A research team at the National Institute of Standards and Technology (NIST) has quantified the interaction of gold nanoparticles with important proteins found in human blood, an approach that should be useful in the development of nanoparticle-based medical therapies and for better understanding the physical origin of the toxicity of certain nanoparticles.
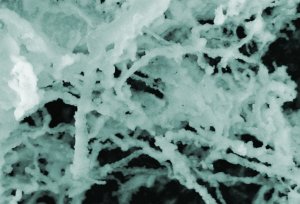 Insulin, one of the most common proteins in human blood, can accumulate into fibrous masses when it misfolds. Research by a team at NIST indicates that gold nanoparticles apparently increase insulin's tendency to form these fibers. (Color added for clarity.) Credit: NIST
Insulin, one of the most common proteins in human blood, can accumulate into fibrous masses when it misfolds. Research by a team at NIST indicates that gold nanoparticles apparently increase insulin's tendency to form these fibers. (Color added for clarity.) Credit: NIST
Nanoparticles show promise as vehicles for drug delivery, as medical diagnostic tools, and as a cancer treatment agent in their own right. Gold nanoparticles, spheres that vary in size between 5 and 100 billionths of a meter in diameter, are especially useful because of the many ways their metal surfaces can be “functionalized” by attaching tailored molecules to perform different tasks in the body. However, treatments require a large number of particles to be injected into the bloodstream, and these could be hazardous if they interact with the body in unforeseen ways.
According to NIST materials scientist Jack Douglas, one of the principal problems confronting nanomedicine is the tendency of proteins to stick to the nanoparticles that float freely in the bloodstream. “Nanoparticles coated with proteins will generally alter their interaction with the body and the nanoparticles can be expected to induce a complementary change in protein chemical activity,” says Douglas. “The coating also can cause the nanoparticles to clump together in large aggregates, which can provoke a huge immune response. Of course, that’s something you want to avoid.”
Scientists have a poor understanding of these interactions, so the NIST team decided to explore what happens when nanoparticles of different sizes encounter five common blood proteins. With the aid of a bevy of microscopes and spectroscopy devices, the team found several general patterns of behavior. “Once the proteins stick to the nanoparticles, the optical properties of both the particles and the proteins change,” Douglas says. “Measuring these changes helps us quantify the stickiness of the nanoparticle for the proteins, the thickness of the adsorbed protein layer and the propensity of the particles to aggregate due to the presence of the protein layers.”
More specifically, the team learned that all five of the proteins stuck to the gold, causing the NPs to aggregate, and that increasing the spheres’ diameter increased their stickiness. They also found that this aggregation usually caused some change in the shape of the proteins—“which generally implies some change in their function as well,” Douglas says.
Aggregation does not always lead to a toxic response, Douglas says, but can affect whether the drugs on the nanoparticles ever reach their intended target. “The main thing is that interactions are largely set by the existence of the protein layer,” he says. “You want to know something about these protein layers if you want to know what nanoparticles are going to do in the body.”
Douglas says that the NIST study addresses metrology needs identified in a National Research Council report** published this past year calling for more quantitative testing for nanoparticle interactions with biological media and that much more work is needed along this and other lines. “For example, we do not yet understand how different-sized particles bind to the surface membranes of cells, which is where many drug interactions take place,” he says.
* S.H.D. Lacerda, J. Park, C. Meuse, D. Pristinski, M.L. Becker, A. Karim and J.F. Douglas. Interaction of gold nanoparticles with common human blood proteins. ACS Nano, December 18, 2009, DOI: 10.1021/nn9011187.
** NRC report, “Review of Federal Strategy for Nanotechnology-Related Environmental, Health, and Safety Research,” available online at www.nap.edu/catalog.php?record_id=12559#toc.