Feb 12 2010
All living cells require a fuel to function: adenosine triphosphate (ATP), the cell "gasoline". Detecting ATP within cells can help researchers observe energetic physiological processes, such as signal cascades or transport processes. Furthermore, ATP depletion is related to certain diseases, such as Parkinson's disease and ischemia (restricted blood flow within tissues). A team led by Michael S. Strano at the Massachusetts Institute of Technology in Cambridge (USA) has now developed a more sensitive, higher-resolution, and more robust method for the detection of ATP. As the scientists report in the journal Angewandte Chemie, the method is based on carbon nanotubes.
 © Wiley-VCH
© Wiley-VCH
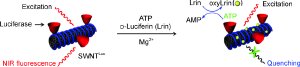
ATP is usually detected by means of the luciferase assay. Luciferases are enzymes that are used in fireflies and other bioluminescent organisms to produce light. They use oxygen to convert a substrate called luciferin into oxyluciferin, which then reacts further to produce light. Certain luciferases use ATP for their reactions. The luciferase assay currently in use is complex, time-consuming, and suffers from a poor signal-to-noise ratio.
The MIT team has now developed a variation of the luciferase protocol: They attached the luciferase to carbon nanotubes. In this form the enzyme is easily taken up by cells. In the presence of luciferin and ATP, oxyluciferin is formed as usual, which causes fluorescence. What is interesting in this case is that carbon nanotubes normally fluoresce in the near infrared (nIR) spectral region; however this is proportionately extinguished by the addition of ATP to the luciferase reaction. Why? "As it is formed, the product oxyluciferin attaches itself firmly to the nanotube," explains Strano. "Electrons are transferred from the nanotube to the oxyluciferin so that the carbon nanotube itself can no longer fluoresce." The reduction in nIR fluorescence is easy to detect and serves as an indicator of the ATP concentration.
"Our new sensor is very selective for ATP," continues Strano. "We were able to use it to observe the change in ATP concentration over time and space in a cell culture."