Aug 16 2010
Clinical trials using patients' own immune cells to target tumors have yielded promising results.
However, this approach usually works only if the patients also receive large doses of drugs designed to help immune cells multiply rapidly, and those drugs have life-threatening side effects.
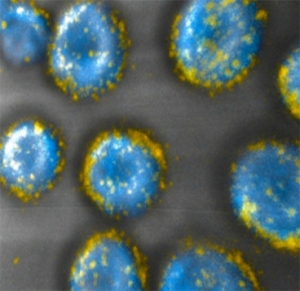 MIT engineers have developed a way to attach drug-carrying pouches (yellow) to the surfaces of cells.
MIT engineers have developed a way to attach drug-carrying pouches (yellow) to the surfaces of cells.
Now a team of MIT engineers has devised a way to deliver the necessary drugs by smuggling them on the backs of the cells sent in to fight the tumor. That way, the drugs reach only their intended targets, greatly reducing the risk to the patient.
The new approach could dramatically improve the success rate of immune-cell therapies, which hold promise for treating many types of cancer, says Darrell Irvine, senior author of a paper describing the technique in the Aug. 15 issue of Nature Medicine.
"What we're looking for is the extra nudge that could take immune-cell therapy from working in a subset of people to working in nearly all patients, and to take us closer to cures of disease rather than slowing progression," says Irvine, associate professor of biological engineering and materials science and engineering and a member of MIT's David H. Koch Institute for Integrative Cancer Research.
The new method could also be used to deliver other types of cancer drugs or to promote blood-cell maturation in bone-marrow transplant recipients, according to the researchers.
To perform immune-cell therapy, doctors remove a type of immune cells called T cells from the patient, engineer them to target the tumor, and inject them back into the patient. Those T cells then hunt down and destroy tumor cells. Clinical trials are under way for ovarian and prostate cancers, as well as melanoma.
Although immune-cell therapy is a promising approach to treating cancer, success has been limited by difficulties in generating enough T cells that are specific to the cancer cells and getting those T cells to function properly in the patient.
To overcome those obstacles, researchers have tried injecting patients with adjuvant drugs that stimulate T-cell growth and proliferation. One class of drugs that has been tested in clinical trials is interleukins — naturally occurring chemicals that help promote T-cell growth but have severe side effects, including heart and lung failure, when given in large doses.
Irvine and his colleagues took a new approach: To avoid toxic side effects, they designed drug-carrying pouches made of fatty membranes that can be attached to sulfur-containing molecules normally found on the T-cell surface.
In the Nature Medicine study, the researchers injected T cells, each carrying about 100 pouches loaded with the interleukins IL-15 and IL-21, into mice with lung and bone marrow tumors. Once the cells reached the tumors, the pouches gradually degraded and released the drug over a weeklong period. The drug molecules attached themselves to receptors on the surface of the same cells that carried them, stimulating them to grow and divide.
Within 16 days, all of the tumors in the mice treated with T cells carrying the drugs disappeared. Those mice survived until the end of the 100-day experiment, while mice that received no treatment died within 25 days, and mice that received either T cells alone or T cells with injections of interleukins died within 75 days.
Irvine's approach to delivering the adjuvant drugs is both simple and innovative, says Dranoff. "The idea of modifying T cells in the lab to make them work better is something many people are exploring through more complicated approaches such as gene modification," he says. "But here, the possibility of just attaching a carefully engineered nanoparticle to the surface of cells could be a much simpler procedure."
While he is now focusing on immune-cell therapy, Irvine believes his cell pouches could be useful for other applications, including targeted delivery of chemotherapy agents. "There are lots of people studying nanoparticles for drug delivery, especially in cancer therapy, but the vast majority of nanoparticles injected intravenously go into the liver or the spleen. Less than 5 percent reach the tumor," says Irvine, who is also a Howard Hughes Medical Institute Investigator.
With a new way to carry drugs specifically to tumors, scientists may be able to resurrect promising drugs that failed in clinical trials because they were cleared from the bloodstream before they could reach their intended targets, or had to be given in doses so high they had toxic side effects.
Irvine and his colleagues also demonstrated that they could attach their pouches to the surface of immature blood cells found in the bone marrow, which are commonly used to treat leukemia. Patients who receive bone-marrow transplants must have their own bone marrow destroyed with radiation or chemotherapy before the transplant, which leaves them vulnerable to infection for about six months while the new bone marrow produces blood cells.
Delivering drugs that accelerate blood-cell production along with the bone-marrow transplant could shorten the period of immunosuppression, making the process safer for patients, says Irvine. In the Nature Medicine paper, his team reports successfully enhancing blood-cell maturation in mice by delivering one such drug along with the cells.
Irvine is now starting to work on making sure the manufacturing process will yield nanoparticles safe to test in humans. Once that is done, he hopes the particles could be used in clinical trials in cancer patients, possibly within the next two or three years.
Source: http://web.mit.edu/