Sep 9 2010
Using a one-of-a-kind instrument designed and built at the National Institute of Standards and Technology (NIST), an international team of researchers have "unveiled" a quartet of graphene's electron states and discovered that electrons in graphene can split up into an unexpected and tantalizing set of energy levels when exposed to extremely low temperatures and extremely high magnetic fields.
Published in this week's issue of Nature, the new research raises several intriguing questions about the fundamental physics of this exciting material and reveals new effects that may make graphene even more powerful than previously expected for practical applications.
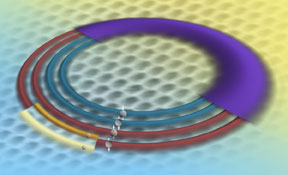 This artist's rendition illustrates the electron energy levels in graphene as revealed by a unique NIST instrument. Because of graphene's properties, an electron in any given energy level (the wide, purple band) comprises four quantum states (the four rings), called a "quartet."
This artist's rendition illustrates the electron energy levels in graphene as revealed by a unique NIST instrument. Because of graphene's properties, an electron in any given energy level (the wide, purple band) comprises four quantum states (the four rings), called a "quartet."
Graphene is one of the simplest materials—a single-atom-thick sheet of carbon atoms arranged in a honeycomb-like lattice—yet it has many remarkable and surprisingly complex properties. Measuring and understanding how electrons carry current through the sheet is important to realizing its technological promise in wide-ranging applications, including high speed electronics and sensors. For example, the electrons in graphene act as if they have no mass and are almost 100 times more mobile than in silicon. Moreover, the speed with which electrons move through graphene is not related to their energy, unlike materials such as silicon where more voltage must be applied to increase their speed, which creates heat that is detrimental to most applications.
To fully understand the behavior of graphene's electrons, scientists must study the material under an extreme environment of ultra-high vacuum, ultra-low temperatures and large magnetic fields. Under these conditions, the graphene sheet remains pristine for weeks, and the energy levels and interactions between the electrons can be observed with precision.
NIST recently constructed the world's most powerful and stable scanning-probe microscope, with an unprecedented combination of low temperature (as low as 10 millikelvin, or 10 thousandths of a degree above absolute zero), ultra-high vacuum and high magnetic field. In the first measurements made with this instrument, the team has used its power to resolve the finest differences in the electron energies in graphene, atom-by-atom.
"Going to this resolution allows you to see new physics," said Young Jae Song, a postdoctoral researcher who helped develop the instrument at NIST and make these first measurements.
And the new physics the team saw raises a few more questions about how the electrons behave in graphene than it answers.
Because of the geometry and electromagnetic properties of graphene's structure, an electron in any given energy level populates four possible sublevels, called a "quartet." Theorists have predicted that this quartet of levels would split into different energies when immersed in a magnetic field, but until recently there had not been an instrument sensitive enough to resolve these differences.
"When we increased the magnetic field at extreme low temperatures, we observed unexpectedly complex quantum behavior of the electrons," said NIST Fellow Joseph Stroscio.
What is happening, according to Stroscio, appears to be a "many-body effect" in which electrons interact strongly with one another in ways that affect their energy levels.
One possible explanation for this behavior is that the electrons have formed a "condensate" in which they cease moving independently of one another and act as a single coordinated unit.
"If our hypothesis proves to be correct, it could point the way to the creation of smaller, very-low-heat producing, highly energy efficient electronic devices based upon graphene," said Shaffique Adam, a postdoctoral researcher who assisted with theoretical analysis of the measurements.
The research team, led by Joseph Stroscio, includes collaborators from NIST, the University of Maryland, Seoul National University, the Georgia Institute of Technology, and the University of Texas at Austin.
The group's work was also recently featured in Nature Physics, in which they describe how the energy levels of graphene's electrons vary with position as they move along the material's crystal structure. The way in which the energy varies suggests that interactions between electrons in neighboring layers may play a role.
Source: http://www.nist.gov/