A team of scientists at the U.S. Department of Energy's (DOE) Argonne National Laboratory and the Carnegie Institution of Washington has succeeded in "watching" nanoparticles grow in real time.
The revolutionary technique allows researchers to learn about the early stages of nanoparticle generation, long a mystery due to inadequate probing methods, and could lead to improved performance of the nanomaterials in applications including solar cells, sensing and more.
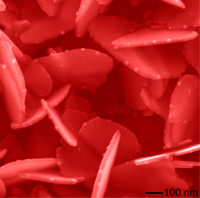 These silver nanoplates are decorated with silver oxy salt nanoparticles along the edges. These nanostructures were grown under irradiation of high-energy x-rays, which allowed scientists to "watch" them grow in real time. The image is from a scanning electron microscope.
These silver nanoplates are decorated with silver oxy salt nanoparticles along the edges. These nanostructures were grown under irradiation of high-energy x-rays, which allowed scientists to "watch" them grow in real time. The image is from a scanning electron microscope.
"Nanocrystal growth is the foundation of nanotechnology," said lead researcher Yugang Sun, an Argonne chemist. "Understanding it will allow scientists to more precisely tailor new and fascinating nanoparticle properties."
The way that nanoparticles look and behave depends on their architecture: size, shape, texture and surface chemistry. This, in turn, depends very much on the conditions under which they are grown.
"Accurately controlling nanoparticles is very difficult," Sun explained. "It's even harder to reproduce the same nanoparticles from batch to batch, because we still don't know all the conditions for the recipe. Temperature, pressure, humidity, impurities—they all affect growth, and we keep discovering more factors."
In order to understand how nanoparticles grow, the scientists needed to actually watch them in the act. The problem was that electron microscopy, the usual method for seeing down into the atomic level of nanoparticles, requires a vacuum. But many kinds of nanocrystals have to grow in a liquid medium—and the vacuum in an electron microscope makes this impossible. A special thin cell allows a tiny amount of liquid to be analyzed in an electron microscope, but it still limited the researchers to a liquid layer just 100 nanometers thick, which is significantly different from the real conditions for nanoparticle synthesis.
To solve this conundrum, Sun found he needed to use the very high-energy X-rays provided at Sector 1 of Argonne’s Advanced Photon Source (APS), which adjoins the laboratory’s Center for Nanoscale Materials, where he works. The pattern of X-rays scattered by the sample allowed the researchers to reconstruct the earliest stages of nanocrystals second-by-second.
"This technique yields a treasure trove of information, especially on the nucleation and growth steps of the crystals, that we had never been able to get before," said Sun.
The intensity of the X-rays does affect the growth of the nanocrystals, Sun said, but the effects only became significant after an especially long reaction time. "Getting a clear image of the growth process will allow us to control samples to get better results, and eventually, new nanomaterials that will have a wide range of applications,” Sun explained.
The nanomaterials could be used in photovoltaic solar cells, chemical and biological sensors and even imaging. For example, noble metal nanoplates can absorb near-infrared light, so they can be used to enhance contrast in images. In one possible case, an injection of specially tailored nanoparticles near a cancer patient's tumor site could increase the imaging contrast between normal and cancerous cells so that doctors can accurately map the tumor.
"The key to this breakthrough was the unique ability for us to work with scientists from the Advanced Photon Source, the Center for Nanoscale Materials and the Electron Microscopy Center—all in one place," Sun said.
Funding for the research was provided by the U.S. Department of Energy's Office of Science. The article, “Nanophase Evolution at Semiconductor/Electrolyte Interface in Situ Probed by Time-Resolved High-Energy Synchrotron X-ray Diffraction”, was published in NanoLetters.
The Center for Nanoscale Materialsat Argonne National Laboratory is one of the five DOE Nanoscale Science Research Centers (NSRCs), premier national user facilities for interdisciplinary research at the nanoscale, supported by the DOE Office of Science. Together the NSRCs comprise a suite of complementary facilities that provide researchers with state-of-the-art capabilities to fabricate, process, characterize and model nanoscale materials, and constitute the largest infrastructure investment of the National Nanotechnology Initiative. The NSRCs are located at DOE’s Argonne, Brookhaven, Lawrence Berkeley, Oak Ridge and Sandia and Los Alamos national laboratories. For more information about the DOE NSRCs, please visit http://nano.energy.gov.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America 's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.