DNA can do more than direct how bodies our made — it can also direct the composition of many kinds of materials, according to a new study from the U.S. Department of Energy’s Argonne National Laboratory.
Argonne researcher Byeongdu Lee and his colleagues at Northwestern University discovered that strands of DNA can act as a kind of nanoscopic "Velcro" that binds different nanoparticles together. "It’s generally difficult to precisely control the assembly of these types of nanostructures," Lee said. "By using DNA, we’re borrowing nature’s power."
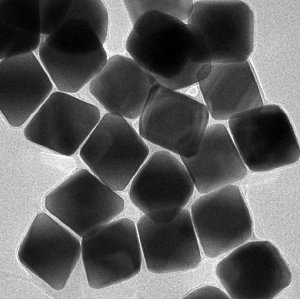 Argonne researcher Byeongdu Lee has determined that different shapes of gold nanoparticles, above and below, will self-assemble into different configurations when attached to single strands of DNA.
Argonne researcher Byeongdu Lee has determined that different shapes of gold nanoparticles, above and below, will self-assemble into different configurations when attached to single strands of DNA.
The "Velcro" effect of the DNA is caused by the molecule’s "sticky ends," which are regions of unpaired nucleotides — the building blocks of DNA — that are apt to bond chemically to their base-pair partners, just like in our genes. When sufficiently similar regions contact each other, chemical bonds form a rigid lattice. Scientists and engineers believe these complex nanostructures have the potential to form the basis of new plastics, electronics and fuels.
In 2008, Lee and his colleagues attached DNA to spherical nanoparticles made of gold, hoping to control the way the particles arrange themselves into compact, ordered crystals. This process is called nanoparticle "packing," and Lee believed that by affixing DNA to the nanoparticles, he could control how they packed together. "Materials that are packed differently — even if they are made from the same substance — have been shown to exhibit dramatically different physical and chemical properties," Lee said.
While the 2008 experiment showed that DNA appeared to control that instance of nanosphere packing, it was not known whether the effect would occur with different nanoparticle geometries. The more recent experiment looked at different shapes of nanoparticles to determine whether their contours affected how they packed.
According to Lee, the spherical nanoparticles in the earlier experiment tended to arrange themselves into one of two separate types of cubic crystals: a face-centered cube (a simple cube with nanospheres at each vertex and additional ones located in the middle of each face) or a body-centered cube (a simple cube with an additional nanosphere located in the middle of the cube itself). The type of lattice that the nanoparticles formed was determined by how the "sticky ends" attached to the nanoparticles paired together.
In the more recent experiment, the particles' shape did change the material's final structure, but only insofar as it altered how the DNA "sticky ends" attached to each other. In fact, the study showed that dodecahedral (12-sided) nanoparticles arranged into a face-centered cubic configuration while octahedral (8-sided) nanoparticles formed body-centered cubes — even when the nanoparticles were attached to identical strands of DNA. "We may be able to make all different types of nanoparticle packing structures, but the structure that will result will always be the one that maximizes the amount of binding," he said.
"The face-centered cubic structure is the most compact way for the nanoparticles to arrange themselves, while the body-centered cubic is slightly less compact. The DNA binding is really the true force controlling the construction of the lattice," he added.
A paper based on the research, "DNA-nanoparticle superlattices formed from anisotropic building blocks", appeared in the October 3 issue of Nature Materials.