Nov 20 2010
For the first time, there is no need to chemically fix, stain or cut cells in order to study them. Instead, whole living cells are fast-frozen and studied in their natural environment.
The new method delivers an immediate 3-D image, thereby closing a gap between conventional microscopic techniques.
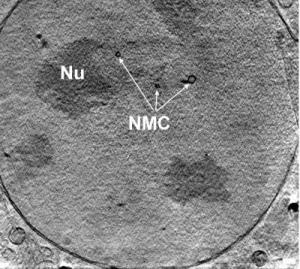 This is a slice through the nucleus of a mouse adenocarcinoma cell showing the nucleolus and the membrane channels running across the nucleus; taken by X-ray nanotomography.
This is a slice through the nucleus of a mouse adenocarcinoma cell showing the nucleolus and the membrane channels running across the nucleus; taken by X-ray nanotomography.
The new microscope delivers a high-resolution 3-D image of the entire cell in one step. This is an advantage over electron microscopy, in which a 3-D image is assembled out of many thin sections. This can take up to weeks for just one cell. Also, the cell need not be labelled with dyes, unlike in fluorescence microscopy, where only the labelled structures become visible. The new X-ray microscope instead exploits the natural contrast between organic material and water to form an image of all cell structures. Dr. Gerd Schneider and his microscopy team at the Institute for Soft Matter and Functional Materials have published their development in Nature Methods (DOI:10.1038/nmeth.1533).
With the high resolution achieved by their microscope, the researchers, in cooperation with colleagues of the National Cancer Institute in the USA, have reconstructed mouse adenocarcinoma cells in three dimensions. The smallest of details were visible: the double membrane of the cell nucleus, nuclear pores in the nuclear envelope, membrane channels in the nucleus, numerous invaginations of the inner mitochondrial membrane and inclusions in cell organelles such as lysosomes. Such insights will be crucial for shedding light on inner-cellular processes: such as how viruses or nanoparticles penetrate into cells or into the nucleus, for example.
This is the first time the so-called ultrastructure of cells has been imaged with X-rays to such precision, down to 30 nanometres. Ten nanometres are about one ten-thousandth of the width of a human hair. Ultrastructure is the detailed structure of a biological specimen that is too small to be seen with an optical microscope.
Researchers achieved this high 3-D resolution by illuminating the minute structures of the frozen-hydrated object with partially coherent light. This light is generated by BESSY II, the synchrotron source at HZB. Partial coherence is the property of two waves whose relative phase undergoes random fluctuations which are not, however, sufficient to make the wave completely incoherent. Illumination with partial coherent light generates significantly higher contrast for small object details compared to incoherent illumination. Combining this approach with a high-resolution lens, the researchers were able to visualize the ultrastructures of cells at hitherto unattained contrast.
The new X-ray microscope also allows for more space around the sample, which leads to a better spatial view. This space has always been greatly limited by the setup for the sample illumination. The required monochromatic X-ray light was created using a radial grid and then, from this light, a diaphragm would select the desired range of wavelengths. The diaphragm had to be placed so close to the sample that there was almost no space to turn the sample around. The researchers modified this setup: Monochromatic light is collected by a new type of condenser which directly illuminates the object, and the diaphragm is no longer needed. This allows the sample to be turned by up to 158 degrees and observed in three dimensions. These developments provide a new tool in structural biology for the better understanding of the cell structure.
Source: http://www.helmholtz.de/