Researchers at the Department of Energy’s Oak Ridge National Laboratory (ORNL) have introduced a unique microscopy method that aids scientific research related to studying reactions that restrict extensive application of fuel cell technologies.
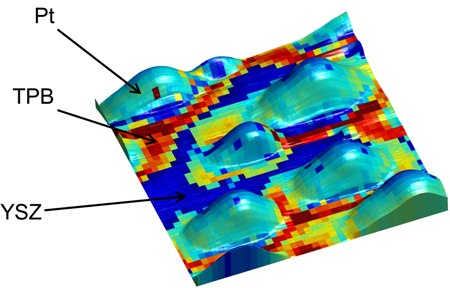 A new ORNL microscopy technique allows researchers to study key reactions in fuel cells at an unprecedented scale. The overlay shows electrochemical activity of platinum (Pt) nanoparticles on an yttria-stabilized zirconia (YSZ) surface, revealing enhanced activity along the triple-phase boundaries (TPB).
A new ORNL microscopy technique allows researchers to study key reactions in fuel cells at an unprecedented scale. The overlay shows electrochemical activity of platinum (Pt) nanoparticles on an yttria-stabilized zirconia (YSZ) surface, revealing enhanced activity along the triple-phase boundaries (TPB).
ORNL research team used a technique known as electrochemical strain microscopy that allowed them to analyze the dynamics of evolution/reduction reactions of oxygen in fuel cell materials, which in turn may disclose ways for cost cutting or redesigning of energy devices. Even though, fuel cells have been considered as an effective way for converting chemical energy into electrical energy, their commercial consumption and production have been restricted due to their high cost. This is because of the utilization of large amount of platinum as a catalyst in the main reaction of the fuel cell, the oxygen-reduction reaction which governs the durability and efficiency of the cell. However, where and how the reaction occurs exactly has not yet been studied because the present electrochemical techniques at device-level are not appropriate for studying the reaction occurring at the nanoscale.
According to Sergei Kalinin, co-author of the study published in Nature Chemistry, specific methods such as electron microscopy have not been successful in studying the fuel cell’s dynamics since their resolution was too high. Several other electrochemical techniques failed to investigate fuel cell’s oxygen-reduction reactions because their resolutions are confined to 10 µm which is larger than a nanometer by about 10,000 times.
Although this research’s main focus is on the application of a technique, scientists represent their method as a pavement for enhancing applied and theoretical exploration of fuel cells. This research was performed at the Center for Nanophase Materials Sciences at ORNL. The co-authors on this study include Anna Morozovska and Francesco Ciucci from the National Academy of Science of Ukrain and the University of Heidelberg respectively.