A research team comprising Hiroshi Handa and his colleagues from the Tokyo Institute of Technology has determined an important protein called cereblon utilizing novel sub-micrometer functionalized magnetic nanobeads developed at the institute.
 Thalidomide treatment or down regulation of the CRBN complex causes developmental defects in zebrafish
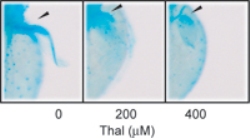
Thalidomide treatment or down regulation of the CRBN complex causes developmental defects in zebrafish
The 200-nm-diameter high-performance affinity magnetic nanobeads are capable of bonding to drugs and other compounds. Cereblon plays a vital role in thalidomide-induced birth defects. The innovation paves the way to develop safer drugs for treating multiple leprosy and myeloma.
The research team utilized ferrite glycidyl methacrylate beads for thalidomide’s purity affiliation, and detected that cereblon as a protein is capable of bonding directly with the medicine. The team then conducted in vivo tests in zebrafish, whose gene is orthologously analogous to cereblon in humans. It studied the growth of embryos wherein this gene was knocked down and same defects were detected as for embryos under the thalidomide treatment. These defects can be avoided by con-injecting messenger RNA for the cereblon-orthologous gene. The findings reveal the possibility of reversing the developmental issues caused by thalidomide treatment.
The team then studied cereblon’s role in chicks. The experiment results confirmed that cereblon was the primary target in birth defects caused by thalidomide treatment. Thalidomide’s action is complicated and is related with several other mechanisms such as antiangiogenic activity and oxidative stress, which may create problems in the development of the fetus.
According to the team, the detection of direct target of thalidomide may be helpful in rationally designing high-efficient thalidomide derivatives with the absence of teratogenic activity. Handa’s description about his work on magnetic particles for biomedicine has been reported in the February issue of the Tokyo Tech Bulletin.