Researchers at Kansas State University and Ohio State University have recorded the vibration of two atoms within a molecule using a superfast camera. The molecular motion was illuminated by using the energy of the electron in the molecule which is similar to a "flash bulb". The study was published in the journal Nature.
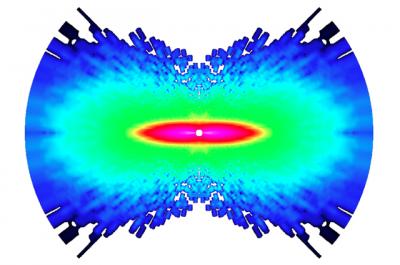 "Researchers at Ohio State University and Kansas State University have captured the first-ever images of atoms moving in a molecule. Shown here is molecular nitrogen. The researchers used an ultrafast laser to knock one electron from the molecule, and recorded the diffraction pattern that was created when the electron scattered off the molecule. The image highlights any changes the molecule went through during the time between laser pulses: one quadrillionth of a second. The constituent atoms' movement is shown as a measure of increasing angular momentum, on a scale from dark blue to pink, with pink showing the region of greatest momentum.Credit: Image courtesy of Cosmin Blaga, Ohio State University."
"Researchers at Ohio State University and Kansas State University have captured the first-ever images of atoms moving in a molecule. Shown here is molecular nitrogen. The researchers used an ultrafast laser to knock one electron from the molecule, and recorded the diffraction pattern that was created when the electron scattered off the molecule. The image highlights any changes the molecule went through during the time between laser pulses: one quadrillionth of a second. The constituent atoms' movement is shown as a measure of increasing angular momentum, on a scale from dark blue to pink, with pink showing the region of greatest momentum.Credit: Image courtesy of Cosmin Blaga, Ohio State University."
In a molecule, ultrafast laser pulses were used to knock out an electron from its own orbit. In analogy to the water wave scattering in the pond or a light flash scattering on a matter, the electron subsequently scattered towards the molecule.
Normally, laser induced electron diffraction (LIED) method is used for solid substances. In this study, the scientists used this method to observe the motion of atoms within a molecule.
Simple molecules like oxygen and nitrogen were selected for the study. The molecules were stroked with quadrillionths of a second or 50 femtoseconds of laser pulses, which knocked out the electron, and the signal scattered through recollission of electron with the molecule was identified.
Louis DiMauro from Ohio State University and the principal investigator stated that the study is the first of its kind to observe chemical reactions with a control in an atomic scale. The findings revealed that the electron’s quantum trajectory can be controlled using the laser pulse adjustment, when it reaches back to the molecule. Steering the electron accurately for controlling a chemical reaction will be the next step of the study.
Cosmin Blaga described that the atoms in the molecules move when the electron is knocked out and recollided with the molecule. The motion of the atoms is captured by a laser induced electron diffraction technique.
Cosmin Blaga, a postdoctoral researcher from Ohio State and DiMauro together with the researchers of Kansas State University conducted the study.