A nanomedicine research project at the David H. Koch Institute for Integrative Cancer Research at Massachusetts Institute of Technology (MIT) has delivered a first-of-its-kind nanomedicine capable of targeting prostate cancer cells and delivering high concentrations of docetaxel chemotherapy in Phase I clinical studies.
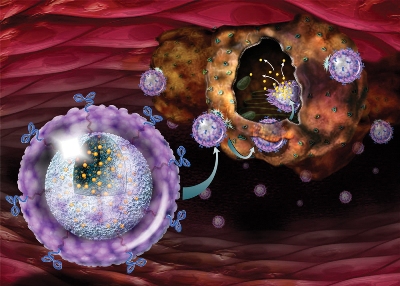 Acting like Trojan horses, targeted nanoparticles can now deliver high doses of anti-cancer compounds, such as chemotherapy agents like docetaxel, directly into prostate cancer cells while sparing healthy cells. (Photo: Business Wire)
Acting like Trojan horses, targeted nanoparticles can now deliver high doses of anti-cancer compounds, such as chemotherapy agents like docetaxel, directly into prostate cancer cells while sparing healthy cells. (Photo: Business Wire)
The direct delivery of the therapeutic utilizing targeted nanoparticles does not cause damage to healthy cells, thus eliminating unwanted side effects. The nanomedicine called BIND-014 was developed by BIND Biosciences, a biopharmaceutical company founded by Robert Langer, a David H. Koch Institute Professor at MIT, and Omid Farokhzad, an Associate Professor at Harvard Medical School. The Prostate Cancer Foundation (PCF) awarded a grant worth $5 million to the project in 2007.
The Phase I clinical study results of BIND-014, BIND Biosciences’ first clinical stage Accurin, have been reported in Science Translational Medicine. BIND Biosciences has also presented the results at the 2012 American Association of Cancer Research meeting conducted in Chicago.
The programmable nanomedicine, BIND-014 integrates a therapeutic nanoparticle and a targeting ligand. It encapsulates docetaxel, a proven anti-cancer drug, in FDA-certified biodegradable and biocompatible polymers. It is designed to target prostate specific membrane antigen (PSMA).
In preclinical cancer models, BIND-014 had demonstrated a ten-fold increase in delivering the quantity of docetaxel to the tumor site when compared to an equivalent dosage of traditional docetaxel, thus enhancing antitumor activity and tolerability. The concept of designing aptamer-targeted nanoparticles was first envisaged in 2002 and developed further by the David H. Koch Institute for Integrative Cancer Research at MIT, Harvard Medical School, the Dana-Farber Cancer Institute, Brigham and Women’s Hospital and Weill Cornell Medical College.
According to Farokhzad, BIND-014’s clinical study results have demonstrated the possibility of developing drugs with both programmable and targeted properties, which allow the direct delivery of therapeutic effect at the disease site, thus transforming the treatment of complex diseases like cancer. These study results will help to safely conduct several Phase I and II studies aimed at other cancers expressing PSMA.