A research team led by Sandra Rosenthal from the Vanderbilt University has discovered a way to improve white-light quantum dots’ fluorescent efficiency up to 45%, a more than 10-fold increase from an initial level of 3%.
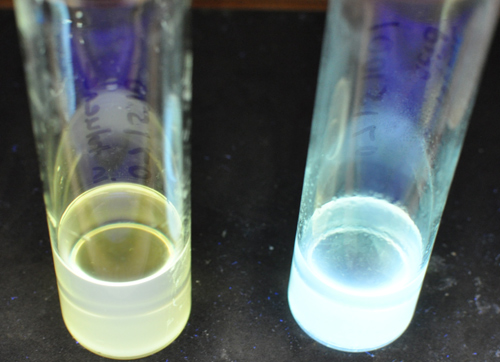 Vial holding original white light quantum dots on the left and the enhanced quantum dots on the right. (Rosenthal Lab)
Vial holding original white light quantum dots on the left and the enhanced quantum dots on the right. (Rosenthal Lab)
A Vanderbilt chemistry lab accidentally discovered the white-light quantum dots seven years ago. What the lab found was ultra-small quantum dots comprising mere 60-70 atoms were able to release white light rather than monochromatic light.
A study performed at the University of North Carolina was the inspiration to the Vanderbilt research. The team first used metal salts to treat the quantum dots for increasing the brightening effect and achieved a 10% to 20% improvement. Since the salts used were acetate salts, the researchers then decided to treat the quantum dots with acetic acid, a carbocyclic acid, which increased the fluorescent efficiency from 8% to 20%.
By taking this as a clue, the researchers then treated the quantum dots with other members of the carbocyclic acid family, and what they discovered was formic acid was able to imprve the fluorescent efficiency up to 45%. Here, the increase in efficiency had changed the peak of the quantum dots’ color spectrum into blue. However, the research team said that it knew the way to fine tune the boosted light’s color balance. The team has plans to test various encapsulating techniques for the enhanced quantum dots.
Rosenthal informed that achieving 45% fluorescent efficiency allows these improved white-light quantum dots to be utilized in certain special lighting applications. This achievement also indicates the possibility of further enhancing the fluorescent efficiency of these nanocrystals.