A research team headed by Hongjie Dai from the Stanford University has synthesized a multi-walled carbon nanotube complex comprising cylindrical carbon sheets, which can be helpful in reducing the cost of catalytic converters, fuel cells, and other energy-related technologies by providing an economical replacement for high-cost platinum catalysts.
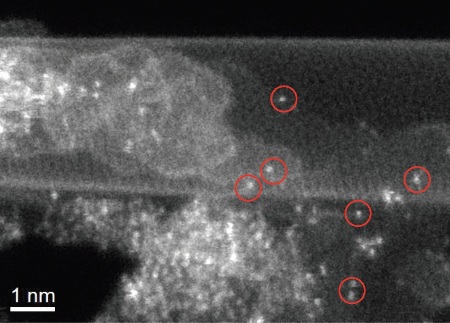 A carbon nanotube complex with promise as a cheap catalyst was thought to have nitrogen and iron impurities that lend the material its desirable chemical properties. Electron microscopy at Oak Ridge National Laboratory confirmed that the material's structure incorporates many heavy atoms, such as the iron atoms circled in red.
A carbon nanotube complex with promise as a cheap catalyst was thought to have nitrogen and iron impurities that lend the material its desirable chemical properties. Electron microscopy at Oak Ridge National Laboratory confirmed that the material's structure incorporates many heavy atoms, such as the iron atoms circled in red.
Seeking for a low-cost substitute, the research team comprising scientists from the Oak Ridge National Laboratory of the Department of Energy selected the abundantly available element, carbon. The novel carbon nanotube material demonstrates catalytic properties analogous to platinum when its outer wall was partly opened by adding ammonia.
While the scientists assumed that the innovative carbon nanotube material’s properties were because of the addition of iron and nitrogen impurities, they were not able to confirm the complex chemical behavior. However, the ORNL microscopic analysis verified that the addition of iron and nitrogen elements to the carbon structure was responsible for the catalytic behavior analogous to that of platinum. The team’s next move is to detect the relationship between iron and nitrogen to identify whether these elements work separately or jointly.
ORNL’s Juan-Carlos Idrobo, one of the researchers, informed that it is difficult to determine elements using traditional transmission electron microscopy. ORNL’s scanning transmission electron microscope uses both spectroscopy and imaging and its powerful nanoscale images directly identify the type of the element. If the element is heavier, then the intensity is brighter. The particular element can be identified with the help of spectroscopy.
The study findings have been reported in the journal, Nature Nanotechnology.