Rensselaer Polytechnic Institute researchers have created a novel graphene anode material that is chargeable and dischargeable at a rate tenfold quicker than traditional graphite anodes utilized in present rechargeable lithium (Li)-ion batteries, without any considerable energy density loss.
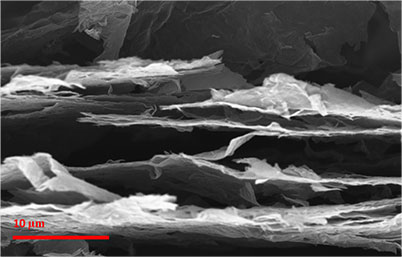 SEM image of the cross section of photo-thermally reduced graphene shows an expanded structure. The graphene sheets are spaced apart with an inter-connected network allowing for greater electrolyte wetting and lithium ion access for efficient high rate performance in lithium ions batteries.
SEM image of the cross section of photo-thermally reduced graphene shows an expanded structure. The graphene sheets are spaced apart with an inter-connected network allowing for greater electrolyte wetting and lithium ion access for efficient high rate performance in lithium ions batteries.
Rechargeable Li-ion batteries have low power density, which affects their rate of charging and discharging. The Rensselaer research team headed by Nikhil Koratkar wanted to solve this issue and develop a new battery that is capable of storing more amount of energy and enables fast charging and discharging. This development paves the way to run electric vehicles only with high-energy, high-power Li-ion batteries, thus eliminating the requirement for complex integration of Li-ion batteries and supercapacitors in them.
To create the graphene anode material, the research team first created a large graphene oxide paper sheet using a known technique. This graphene paper with a thickness of a normal printer paper can be produced in almost any shape or size. Some of these graphene oxide papers were then exposed to a digital camera flash and other samples were exposed to a laser. In both cases, the heat generated from the photoflash or laser triggered mini-explosions all across the paper due to the release of oxygen atoms in graphene oxide, creating myriad voids, pores, cracks and other blemishes.The thickness of the graphene paper was also increased to five folds because of the buildup of pressure due to the release of oxygen atoms. Thus, large voids created between the individual graphene sheets.
The research team quickly discovered that this defect-engineered graphene paper was able to better perform as a Li-ion battery anode as the pores and cracks created on the paper allows the lithium ions to traverse quickly across the length of graphene sheets, thus increasing overall power density of the battery and enabling faster charging and discharging. The team demonstrated the robustness of its graphene anode material that performed well even after over 1,000 charge/discharge cycles. The graphene sheet’s high electrical conductivity caused effective electron transportation in the anode, which is also an important aspect for high-power applications.
The researchers’ next step is to couple the graphene anode material with a superior-power cathode material to build a complete battery.