Nov 28 2012
Now, Helmholtz Centre Berlin's Dr. Andrei Varykhalov, Prof. Dr. Oliver Rader and his team of physicists has taken the first step towards building graphene-based components, in collaboration with physicists from St. Petersburg (Russia), Jülich (Germany) and Harvard (USA). According to their report on 27. November 2012 in Nature Communications (DOI: 10.1038/ncomms2227), they successfully managed to increase the graphene conduction electrons' spin-orbit coupling by a factor of 10,000 – enough to allow them to construct a switch that can be controlled via small electric fields.
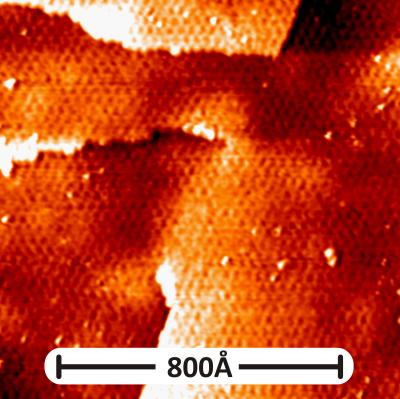 Scanning tunneling microscopy shows the topography of graphene on gold with periodic beatings ten times larger than the periodicity of the carbon atoms. These beatings are moiré-patterns, emerging because of the different atomic structures of graphene and the underlying monolayer of gold atoms. The structure of moiré influences chemical interactions between gold and graphene layer and also electronic properties and spin behavior in graphene. (Credit: HZB/Andrei Varykhalov)
Scanning tunneling microscopy shows the topography of graphene on gold with periodic beatings ten times larger than the periodicity of the carbon atoms. These beatings are moiré-patterns, emerging because of the different atomic structures of graphene and the underlying monolayer of gold atoms. The structure of moiré influences chemical interactions between gold and graphene layer and also electronic properties and spin behavior in graphene. (Credit: HZB/Andrei Varykhalov)
The graphene layer sits on top of a nickel substrate whose atoms are separated by the same distance as graphene's hexagonal meshes. Next, the physicists deposited gold atoms on their sample that ended up lodging between the graphene and the nickel.
Using different photoelectron spectrometers at HZB's own BESSY II synchrotron radiation facility allowed the researchers to measure changes in graphene's electronic properties. Just like the earth, electrons have two angular momenta: an orbital angular momentum, which allows them to circle the atomic nucleus; and a spin corresponding to a rotation about their own axes. A strong spin-orbit coupling thus means a big energetic difference depending on whether both rotations are directed in the same or in opposite directions. In the case of lighter nuclei (as is true for carbon atoms), the spin-orbit interaction is rather weak, whereas in the case of heavier atoms like gold it is quite strong. "We could show that, given their proximity to the graphene layer, the gold atoms were also able to increase this interplay in the graphene layer by a factor of 10,000," explains Dmitry Marchenko who took the measurements as part of his Ph.D. research.
According to Varykhalov, this very strong spin-orbit coupling would allow the researchers to build a switch of sorts as the spins could now be rotated using an electric field. Two spin filters – one in front of and one behind the component – would each tolerate unidirectional spins only. If the spin filters were perpendicular to each other, no spin would be able to get through anymore and the switch would be effectively shut off. An electric field, however, would rotate the spins in such a way that it would be able to – partially or completely – turn up the switch.
"We were able to document that only electrons in the 5d orbitals of gold atoms increase graphene's spin orbit interaction. This conforms to our theoretical models," explains Varykhalov. Nonetheless, the HZB physicists have their next challenge cut out for themselves already: a graphene-based component that sits on a non-conducting surface instead of nickel, a metal. Not surprisingly, they have already begun working on it.