A research group at the National Institute of Standards and Technology (NIST) has developed a relatively simple, fast and effective method of depositing uniform, ultrathin layers of platinum atoms on a surface.* The new process exploits an unexpected feature of electrodeposition of platinum—if you drive the reaction much more strongly than usual, a new reaction steps in to shuts down the metal deposition process, allowing an unprecedented level of control of the film thickness.
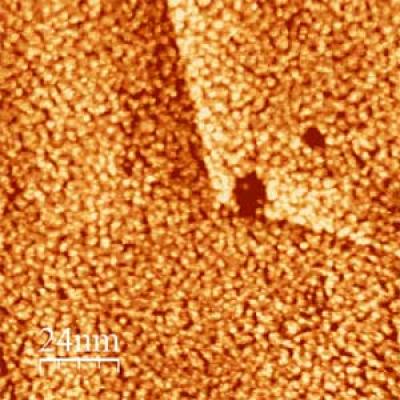 Scanning tunneling microscope image shows ultrathin film layer of platinum deposited on gold after five seconds, using the NIST process. Darker areas are exposed gold substrate not yet covered by the platinum. (Credit: Gokcen/NIST)
Scanning tunneling microscope image shows ultrathin film layer of platinum deposited on gold after five seconds, using the NIST process. Darker areas are exposed gold substrate not yet covered by the platinum. (Credit: Gokcen/NIST)
Platinum is a widely used industrial catalyst—in automobile catalytic converters and hydrogen fuel cells—as well as a key component in microelectronics, so the discovery may have widespread application in the design and manufacture of platinum-based devices.
The metal is rare, and hence very pricey, so materials engineers try to use it sparingly as a thin layer on a substrate. They'd like to be able to control the deposition process down to uniform, single layers of atoms. Unfortunately, platinum doesn't always cooperate.
The model system studied at NIST—depositing a platinum layer on gold by electroplating—demonstrates the challenging nature of the problem. A voltage is applied to drive the deposition of platinum from an electrode onto the gold surface in an aqueous solution. Normally, this leads to a patchy and rough surface rather than the desired smooth and even layer of platinum, because platinum tends to attach first to any defects on the gold surface, and then tends to attach to itself, rather than the gold.
The NIST team has found that increasing the voltage, the driving force of the reaction, far higher than normal to the point where the water molecules start to break down and hydrogen ions form, leads to an unexpected and useful result. The hydrogen quickly forms a layer covering the freshly deposited platinum islands and completely quenches further metal deposition. Using a battery of analytic techniques, including a quartz crystal microbalance, X-ray photoelectron spectroscopy and scanning tunneling microscopy, the group found that the formation of the hydrogen layer was rapid enough to restrict deposition to the formation of a single layer of platinum atoms. The team further discovered that by pulsing the applied voltage, they could selectively remove the hydrogen layer to enable the platinum deposition process to be repeated to form another layer.
The deposition process occurs in a single plating bath and is surprisingly fast—1,000 times faster than making comparable films using molecular beam epitaxy, for example. It's also faster, simpler and less prone to contamination than other electrochemical techniques for depositing platinum films, making it much less expensive.