A class of water-loving, jelly-like materials with uses ranges ranging from the mundane, such as superabsorbent diaper liners, to the sophisticated, such as soft contact lenses, could be tapped for a new line of serious work: testing the biological effects of nanoparticles now being eyed for a large variety of uses.
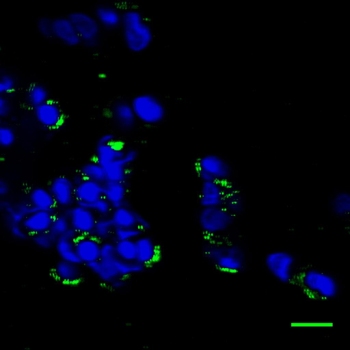 After four days, quantum dots still shine (green) in cells embedded in a hydrogel scaffold. Credit: Mansfield/NIST
After four days, quantum dots still shine (green) in cells embedded in a hydrogel scaffold. Credit: Mansfield/NIST
New research* by scientists at the National Institute of Standards and Technology (NIST) demonstrates that three-dimensional scaffolds made with cells and supporting materials known as hydrogels can serve as life-like measurement platforms for evaluating how tiny engineered materials interact with cells and tissues. Their proof-of-concept study suggests that hydrogel tissue scaffolds can be a "powerful bridge" between current laboratory tests and tests that use animal models.
Today, laboratory tests of nanoparticles usually entail exposing a two-dimensional layer of cells to the material of interest. Besides being questionable substitutes for the complex cellular frameworks that make up tissues and organs inside the body, these tests can yield conflicting results, explains analytical chemist Elisabeth Mansfield, lead researcher on the new NIST study.
"Our study shows that hydrogel-based, tissue-engineering scaffolds can provide more realistic environments to study nanoparticle-influenced cell biology over extended periods," she says. Importantly, the NIST research shows that studies employing the scaffold do not require exposing cells to nanoparticles in doses that exceed normal exposure levels.
Hydrogels are networks of stringy, branching polymer molecules with ends that latch onto water molecules—so much so that 99.9 percent of a hydrogel may consist of water. Depending on the spacing between the strands (the so-called mesh size) and other factors, hydrogels can support and promote the growth and differentiation of cell populations.
While hydrogels occur naturally—an example is cartilage—the NIST team chose to craft its own, giving them control over the mesh size in the scaffolds they created.
In their experiment, the team used polyethylene glycol—a common polymer used in skin creams, toothpaste, lubricants and other products—to create three hydrogels with different mesh sizes. One set of hydrogels was populated with rat cells containing ultrasmall semiconducting materials known as quantum dots. When exposed to light, quantum dots emit strong fluorescent signals that enabled the researchers to track the fate of treated cells in the synthetic scaffolds.
Results were compared with those for similarly treated cells grown in a single layer on a substrate, akin to standard laboratory toxicology tests.
The NIST researchers found that cells diffused through the hydrogel scaffold, forming a persisting tissue-like structure. Quantum dots attached to cell membranes and, over time, were absorbed into the cells.
Three-dimensional scaffolds often are used to test cells for multi-week experiments, and NIST researchers found quantum dots can be detected for four or more days inside the scaffold.
As significant, cells that populated the hydrogel scaffolds were exposed to lower levels of quantum dots, yielding a more representative scenario for evaluating biological effects.
The NIST team concludes that, compared with conventional cell cultures, hydrogel scaffolds provide a more realistic, longer-lived biological environment for studying how engineering nanoparticles interact with cells. In addition, the scaffolds will accommodate studies of how these interactions evolve over time and of how the physical features of nanoparticles may change.