Minuscule crystals that glow different colors may be the missing ingredient for white LED lighting that illuminates homes and offices as effectively as natural sunlight.
 Oak Ridge National Laboratory scientists are using x-ray diffraction analysis to better understand tiny crystals that could be used in warm-white LEDs.
Oak Ridge National Laboratory scientists are using x-ray diffraction analysis to better understand tiny crystals that could be used in warm-white LEDs.
Light-emitting diodes, better known as LEDs, offer substantial energy savings over incandescent and fluorescent lights and are easily produced in single colors such as red or green commonly used in traffic lights or children's toys.
Developing an LED that emits a broad spectrum of warm white light on par with sunlight has proven tricky, however. LEDs, which produce light by passing electrons through a semiconductor material, often are coupled with materials called phosphors that glow when excited by radiation from the LED.
"But it's hard to get one phosphor that makes the broad range of colors needed to replicate the sun," said John Budai, a scientist in ORNL's Materials Science and Technology division. "One approach to generating warm-white light is to hit a mixture of phosphors with ultraviolet radiation from an LED to stimulate many colors needed for white light."
Budai is working with a team of scientists from University of Georgia and Oak Ridge and Argonne national laboratories to understand a new group of crystals that might yield the right blend of colors for white LEDs as well as other uses. Zhengwei Pan's group at UGA grew the nanocrystals using europium oxide and aluminum oxide powders as the source materials because the rare-earth element europium is known to be a dopant, or additive, with good phosphorescent properties.
"What's amazing about these compounds is that they glow in lots of different colors—some are orange, purple, green or yellow," Budai said. "The next question became: why are they different colors? It turns out that the atomic structures are very different."
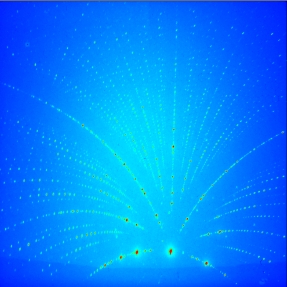
Budai has been studying the atomic structure of the materials using x-rays from Argonne's Advanced Photon Source. Two of the three types of crystal structures in the group of phosphors had never been seen before, which can probably be attributed to the crystals' small size, Budai said.
"Only the green ones were a known crystal structure," Budai said. "The other two, the yellow and blue, don't grow in big crystals; they only grow with these atomic arrangements in these tiny nanocrystals. That's why they have different photoluminescent properties."
X-ray diffraction analysis is helping Budai and his collaborators work out how the atoms are arranged in each of the different crystal types. The different-colored phosphors exhibit distinct diffraction patterns when they are hit with x-rays, enabling researchers to analyze the crystal structure.
"What that means in terms of how the electrons around the atoms interact to make light is much harder," Budai said. "We haven't completely solved that yet. That's the continuing research. We have a lot of clues, but we don't know everything."
The knowledge gained through their atomic-scale analysis is helping the research team improve the phosphorescent crystals. Different factors in the growth process—temperature, powder composition, and types of gas used—can change the final product. A fundamental understanding of all the parameters could help the team to perfect the recipe and improve the crystals' ability to convert energy into light.
Advancing the material's luminescence efficiency is key to making it useful for commercial LED products and other applications; the new nanocrystals may turn out to have other practical photonic uses beyond phosphors for LEDs. Their ability to act as miniature "light pipes" when the crystal quality is high enough could lend them to applications in fiber-optic technologies, Budai said.
"You can keep growing the crystals and measuring them, or you can understand why it's doing what it's doing, and figure out how to make it better. That's what we're doing—basic research. We have to figure out nature first."
The team's most recent study is published as the inside front cover article in the April 25 issue of Advanced Functional Materials as "New Ternary Europium Aluminate Luminescent Nanoribbons for Advanced Photonics."
Budai and use of the Advanced Photon Source at Argonne were supported by DOE's Office of Science. Zhengwai Pan was funded by the National Science Foundation.
The Advanced Photon Source at Argonne National Laboratory is one of five national synchrotron radiation light sources supported by the U.S. Department of Energy's Office of Science to carry out applied and basic research to understand, predict, and ultimately control matter and energy at the electronic, atomic, and molecular levels, provide the foundations for new energy technologies, and support DOE missions in energy, environment, and national security. To learn more about the Office of Science X-ray user facilities, visit http://science.energy.gov/user-facilities/basic-energy-sciences/.