NANOBIOTIX, a clinical-stage nanomedicine company pioneering novel approaches for the local treatment of cancer, announces the selection of a new product for development, NBTX-IV. It is the second product of the NanoXray pipeline which is designed for systemic administration (intravenous injection).
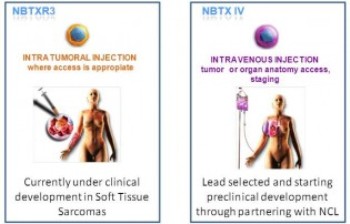
This product has been selected by the National Cancer Institute's (NCI) Nanotechnology Characterization Laboratory (NCL) for characterization on the basis of its potential to impact cancer treatment.
NBTX-IV is based on Nanobiotix's proprietary NanoXray platform. It is designed to be administered intravenously to target deep-seated tumors and lymph nodes which may have been invaded locally by cancer cells. The product aims to enhance radiotherapy energy to destroy cancer cells and reduce the subsequent escape of malignant cells localized in neighboring tissues cells or lymph nodes. Target indications include lung carcinoma, pancreatic cancer or brain metastases.
As part of the collaboration with NCI, the NCL will perform pre-clinical characterization of NBTX-IV that will support Nanobiotix's filing of an Investigational New Drug (IND) with the FDA. In parallel, Nanobiotix will conduct additional preclinical testing to provide a complete dossier for submission.
The NCL was established to investigate the use of nanoparticulate material for the advancement of cancer research and to accelerate the development of promising and safe nanotechnology-derived cancer therapeutics. It provides preclinical testing and consultation services on a competitive basis to developers, like Nanobiotix, and is working in concert with other US agencies such as the U.S. Food and Drug Administration (FDA) to accelerate the transition of basic nanomedicine research into clinical applications.
"The selection of a second NanoXray product for development coupled with the characterization being undertaken by the NCL is an important strategic step for Nanobiotix," said Laurent Levy, CEO of Nanobiotix. "This collaboration with the NCL will hopefully accelerate the pre-clinical development of NBTX-IV, opening the pathway for new clinical indications in addition to the one covered by our first product. The results of the characterization are not only important for our future IND submission, but also raise our profile in the US market."