Jul 24 2013
A simple, low-cost and eco-friendly method of creating nitrogen-doped graphene nanoplatelets (NGnPs), which could be used in dye-sensitized solar cells and fuel cells, is published in Scientific Reports today.
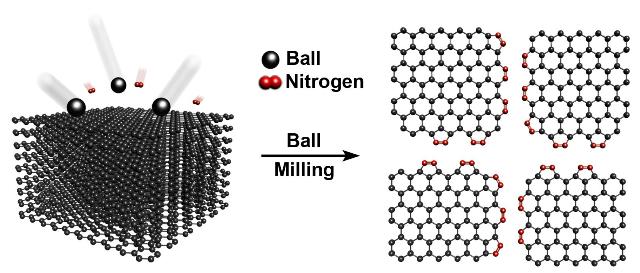 Diagram of nitrogen-goped graphene nanoplatelets (NGnPs)
Diagram of nitrogen-goped graphene nanoplatelets (NGnPs)
The work, carried out at Ulsan National Institute of Science and Technology (UNIST) in South Korea, could be a step towards replacing conventional platinum (Pt)-based catalysts for energy conversion.
The search for economically viable alternatives to fossil fuels has attracted attention among energy communities because of increasing energy prices and climate change. Solar cells and fuel cells are to be promising alternatives, but Pt-based electrodes are expensive and susceptible to environmental damage.
Nitrogen fixation is where nitrogen (N2) in the atmosphere is converted into ammonia (NH3). Fixation processes free up nitrogen atoms from their diatomic form to be used in other ways, but nitrogen does not easily react with other chemicals to form new compounds.
The most common method of industrial nitrogen fixation is the Harber-Bosch process, which requires extremely harsh conditions, 200 atm of pressure and 400 °C of temperature.
The UNIST team previously reported that dry ball-milling can efficiently produce chemically modified graphene particles in large quantities*. This research, in Scientific Reports, presents another innovation to improve the materials. Along the way, the research team discovered a novel nitrogen fixation process.
They focus on modifications with nitrogen, developing a technique with direct nitrogen fixation, carbon-nitrogen bond formation, at the broken edges of graphite frameworks using ball-milling graphite in the presence of nitrogen gas.
The research was led by Jong-Beom Baek, professor and director of the Interdisciplinary School of Green Energy/Low-Dimensional Carbon Materials Center, UNIST, Liming Dai, professor of Case Western Reserve University and Noejung Park, professor of the Interdisciplinary School of Green Energy, UNIST.
“Nitrogen is the most abundant constituent in air and it is inert diatomic gas while graphite is the most thermodynamically stable form of carbon allotropes,” said Prof. Baek. “It is an extreme challenge for the C-N bond formation directly from graphite and nitrogen.”
This research was supported by World Class University (WCU), US-Korea NBIT, Mid-Career Researcher (MCR), Converging Research Center (CRC) and Basic Research Laboratory (BRL) programs through the National Research Foundation (NRF), of Korea funded by the Ministry of Science, ICT and Future Planning (Minister Choi Mun-Kee), US Air Force Office of Scientific Research through Asian Office of Aerospace R&D (AFOSR-AOARD), and AFOSR.