Jul 29 2013
When University of Pennsylvania nanoscientists created beautiful, tiled patterns with flat nanocrystals, they were left with a mystery: why did some sets of crystals arrange themselves in an alternating, herringbone style, even though it wasn’t the simplest pattern? To find out, they turned to experts in computer simulation at the University of Michigan and the Massachusetts Institute of Technology.
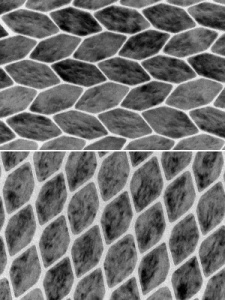 These transmission electron microscope images show the two different patterns the nanocrystals could be made to pack in.
These transmission electron microscope images show the two different patterns the nanocrystals could be made to pack in.
The result gives nanotechnology researchers a new tool for controlling how objects one-millionth the size of a grain of sand arrange themselves into useful materials, it gives a means to discover the rules for “programming” them into desired configurations.
The study was led by Christopher Murray, a professor with appointments in the Department of Chemistry in the School of Arts and Sciences and the Department of Materials Science and Engineering in the School of Engineering and Applied Sciences. Also on the Penn team were Cherie Kagan, a chemistry, MSE and electrical and systems engineering professor, and postdoctoral researchers Xingchen Ye, Jun Chen and Guozhong Xing.
They collaborated with Sharon Glotzer, a professor of chemical engineering at Michigan, and Ju Li, a professor of nuclear science and engineering at MIT.
Their research was featured on the cover of the journal Nature Chemistry.
“The excitement in this is not in the herringbone pattern,” Murray said, “It’s about the coupling of experiment and modeling and how that approach lets us take on a very hard problem.”
Previous work in Murray’s group has been focused on creating nanocrystals and arranging them into larger crystal superstructures. Ultimately, researchers want to modify patches on nanoparticles in different ways to coax them into more complex patterns. The goal is developing “programming matter,” that is, a method for designing novel materials based on the properties needed for a particular job.
“By engineering interactions at the nanoscale,” Glotzer said, “we can begin to assemble target structures of great complexity and functionality on the macroscale.”
Glotzer introduced the concept of nanoparticle “patchiness” in 2004. Her group uses computer simulations to understand and design the patches.
Recently, Murray’s team made patterns with flat nanocrystals made of heavy metals, known to chemists as lanthanides, and fluorine atoms. Lanthanides have valuable properties for solar energy and medical imaging, such as the ability to convert between high- and low-energy light.
They started by breaking down chemicals containing atoms of a lanthanide metal and fluorine in a solution, and the lanthanide and fluorine naturally began to form crystals. Also in the mix were chains of carbon and hydrogen that stuck to the sides of the crystals, stopping their growth at sizes around 100 nanometers, or 100 millionths of a millimeter, at the largest dimensions. By using lanthanides with different atomic radii, they could control the top and bottom faces of the hexagonal crystals to be anywhere from much longer than the other four sides to non-existent, resulting in a diamond shape.
To form tiled patterns, the team purified the nanocrystals and mixed them with a solvent. They spread this mixture in a thin layer over a thick fluid, which supported the crystals while allowing them to move. As the solvent evaporated, the crystals had less space available, and they began to pack together.
The diamond shapes and the very long hexagons lined up as expected, the diamonds forming an argyle-style grid and the hexagons matching up their longest edges like a foreshortened honeycomb. The hexagons whose sides were all nearly the same length should have formed a similar squashed honeycomb pattern, but, instead, they lined up in an alternating herringbone style.
“Whenever we see something that isn’t taking the simplest pattern possible, we have to ask why,” Murray said.
They posed the question to Glotzer’s team.
“They’ve been world leaders in understanding how these shapes could work on nanometer scales, and there aren’t many groups that can make the crystals we make,” Murray said. “It seemed natural to bring these strengths together.”
Glotzer and her group built a computer model that could recreate the self-assembly of the same range of shapes that Murray had produced. The simulations showed that if the equilateral hexagons interacted with one another only through their shapes, most of the crystals formed the foreshortened honeycomb pattern, not the herringbone.
“That’s when we said, ‘Okay, there must be something else going on. It’s not just a packing problem,’” Glotzer said. Her team, which included graduate student Andres Millan and research scientist Michael Engel, then began playing with interactions between the edges of the particles. They found that that if the edges that formed the points were stickier than the other two sides, the hexagons would naturally arrange in the herringbone pattern.
The teams suspected that the source of the stickiness was those carbon and hydrogen chains. Perhaps they attached to the point edges more easily, the team members thought. Since experiment doesn’t yet offer a way to measure the number of hydrocarbon chains on the sides of such tiny particles, Murray asked MIT’s Ju Li to calculate how the chains would attach to the edges at a quantum mechanical level.
Li’s group confirmed that, because of the way that the different facets cut across the lattice of the metal and fluorine atoms, more hydrocarbon chains could stick to the four edges that led to points than the remaining two sides. As a result, the particles become patchy.
“Our study shows a way forward making very subtle changes in building block architecture and getting a very profound change in the larger self-assembled pattern,” Glotzer said. “The goal is to have knobs that you can change just a little and get a big change in structure, and this is one of the first papers that shows a way forward for how to do that.”
The research was supported by the Office of Naval Research, the National Science Foundation, the U.S. Department of Energy and the U.S. Department of Defense.
Murray is a Penn Integrates Knowledge professor. The PIK program is an initiative conceived by President Amy Gutmann to recruit scholars whose research and teaching exemplify the integration of knowledge. These scholars hold endowed professorships and joint appointments between Penn's schools.