Dec 11 2013
As embryonic tissue develops, cells push and pull on each other, and they must do so correctly for the tissue to develop properly. Now scientists at Harvard University have devised the first method to measure these forces in three-dimensional (3D) tissues and living embryos.
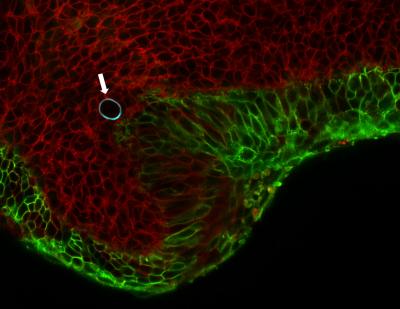 This image shows cells in the embryonic jaw of a mouse (red) squeeze a cell-sized, oil droplet (with blue border, shown with arrow). The droplet deforms like a water balloon, and the degree to which it deforms allows scientists to calculate the pressure cells exert on their neighbors. Credit: Otger Campas/Nature Methods.
This image shows cells in the embryonic jaw of a mouse (red) squeeze a cell-sized, oil droplet (with blue border, shown with arrow). The droplet deforms like a water balloon, and the degree to which it deforms allows scientists to calculate the pressure cells exert on their neighbors. Credit: Otger Campas/Nature Methods.
The method, which involves injecting tiny oil droplets, could lead to new tools to diagnose cancer, hypertension, connective tissue diseases, and more. Scientists from the Wyss Institute for Biological Engineering at Harvard University and the Harvard School of Engineering and Applied Sciences (SEAS) reported the work online December 8 in Nature Methods.
"Now that we can quantitate cellular forces, we can find entirely new ways to diagnose the extraordinarily wide range of diseases that alter cell contractility and tissue stiffness," said Don Ingber, M.D., Ph.D., Founding Director of the Wyss Institute, Professor of Bioengineering at SEAS, and senior author of the study. "Just as important, we can answer crucial questions about development that have lay dormant for decades."
Biological tissues don't just sit there inside the body — they are constantly in motion, with cells tugging on and nudging other cells and the extracellular matrix — the molecular scaffold that knits cells together into tissues. As a result, tissues live in a state of dynamic tension, like a partially stretched rubber band.
Studies in lab-grown cells suggest that mechanical forces are as important in regulating biological function as chemicals and genes. But scientists had no way of studying those control mechanisms in developing embryos because they had no way of quantifying mechanical forces at specific positions in living tissues.
Such forces are particularly important as the body develops from the fertilized egg into tissues and organs with specialized shapes and functions — a process known as morphogenesis. Biologists studying morphogenesis knew that as the embryo develops, mechanical forces direct cells to multiply, steer to their proper locations, and specialize. But they have long focused on detailing the genes and cellular pathways that direct and coordinate this process, rather than the role of cellular forces — simply because they did not have the tools to measure those forces, said Otger Campàs, Ph.D., an Assistant Professor of mechanical engineering who holds the Mellichamp Chair in Systems Biology at the University of California, Santa Barbara (UCSB). Campàs is a former postdoctoral fellow at the Wyss Institute and SEAS.
"Shaping tissues and organs involves an interplay between genetics and physics. If you can't measure the physical side of it, you can't completely understand the problem," Campàs said.
Scientists had previously developed several methods to quantitate how cells push and pull on each other while growing in a dish in the lab. But they had no good way to measure these forces while the cells are building 3D tissues in their natural environment.
Campàs decided to invent one. As a doctoral student, he had used oil droplets to measure forces exerted by a network of protein filaments that drive cell movement. Inspired by that work, he decided to try using oil microdroplets as force transducers in living tissues.
Campàs and Ingber identified a special oil called a fluorocarbon that remains separate from the cell membrane, like oil does from water, and is safe for cells and tissues. Then they devised a special coating for the droplets so it sticks to cells or to the extracellular matrix. This enabled them to measure how cells push and pull within living tissues.
They also coated droplets with a chemical that made their surface glow when illuminated with a laser, then videotaped under a microscope as cells tugged and pressed on the droplet in 3D. The oil droplets on their own are spherical, but squeezing or stretching them deforms them as if squeezing or stretching a water balloon. By measuring how deformed each droplet was, the scientists could precisely calculate the force exerted on it by the neighboring cells adhering to it and by the extracellular matrix.
Using the new method, the scientists were able to quantitate the forces within lab-grown 3D aggregates of mouse mammary tumor cells, and within living 3D tissues from embryonic mouse jaws. They found that an individual cell exerted huge forces — 24 times as much pressure on the droplet as the jaws of an ant – and that the cells exerted the same amount of force in cultured aggregates as in tissues, lending confidence to the method's accuracy.
In his new lab at UCSB, Campàs now uses the method to determine the spatial patterns of forces that shape different embryonic structures in fish, chicken, and other organisms.