Jan 31 2014
Plasmonic nanoparticles developed at Rice University are becoming known for their ability to turn light into heat, but how to use them to generate electricity is not nearly as well understood.
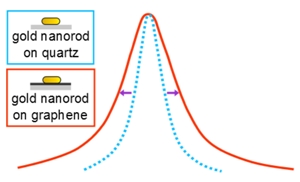 The plasmon resonance of gold nanorods on graphene is broadened compared with gold nanorods on quartz, according to a new study by Rice University scientists. The additional peak width was attributed to excited electron transfer between gold nanorods and graphene. (Credit: Anneli Hoggard/Rice University)
The plasmon resonance of gold nanorods on graphene is broadened compared with gold nanorods on quartz, according to a new study by Rice University scientists. The additional peak width was attributed to excited electron transfer between gold nanorods and graphene. (Credit: Anneli Hoggard/Rice University)
Scientists at Rice are working on that, too. They suggest that the extraction of electrons generated by surface plasmons in metal nanoparticles may be optimized.
Rice researchers led by chemist Stephan Link and graduate student Anneli Hoggard are endeavoring to understand the physics; they started by measuring the speed and efficiency of excited “hot” electrons drawn from gold nanoparticles into a sheet of graphene.
It’s a good thing for scientists and engineers to know as they work on technologies beyond standard photovoltaic devices that gobble light to drive chemical reactions or next-generation electronics. The work was reported recently in the American Chemical Society journal ACS Nano.
“We’ve looked at this process on a single-particle level,” said lead author Hoggard. “Instead of looking at a device that has many junctions, we’ve looked at one particle at a time. We had to measure a lot of particles to get good statistics.”
Dark-field scattering and photoluminescence spectroscopy of more than 200 nanoparticles helped them determine that it takes about 160 femtoseconds (quadrillionths of a second) for an electron to transfer from the particle to highly conducting graphene, the single-atom-thick form of carbon.
Plasmons are the collective excitation of free electrons in metals that, when stimulated by an energy source like sunlight or a laser, set up a harmonic oscillation of the surface charges similar to waves. In the process, they scatter light that can be read by a spectrometer, which captures and categorizes light according to its wavelengths.
If the energy input is intense enough, the output can be intense as well. In one practical example demonstrated at Rice, plasmon excitation in gold nanoparticles produces heat that instantly turns even ice-cold water into steam.
That excitation energy can also be channeled in other directions through the creation of hot electrons that can transfer to suitable acceptors, Link said, but how fast usable electrons flow from plasmonic nanoparticles is little understood. “The plasmon generates hot electrons that decay very quickly, so intercepting them is a challenge,” he said. “We’re now realizing these electrons can be useful.”
That thought prompted Link’s lab to embark upon the painstaking effort to analyze single nanoparticles. The researchers placed gold nanorods on beds of both inert quartz and highly conductive graphene and used a spectrometer to view the line width of the plasmon-scattering spectrum.
The homogeneous line width obtained via single-particle spectroscopy is a measure of the range of wavelengths that resonantly excite a surface plasmon. It’s also a measure of the plasmon lifetime. Broad line widths correspond to short lifetimes and narrow line widths to long lifetimes.
The Rice researchers found graphene broadened the nanorods’ surface plasmon response – and shortened its lifetime – by accepting hot electrons. By acting as an electron acceptor, the graphene accelerated damping of the plasmons. The difference in damping between the quartz and graphene samples provided a means to calculate the electrons’ transfer time.
“The plasmon resonance is determined by the size and the shape of the nanoparticle,” Hoggard said. “And it usually appears as a single peak for gold nanorods. But there are important parameters about the peak: The position and the width of the peak can give us information about the particle itself, or the type of environment it’s in. So we looked at how the width of the peak changes when nanoparticles are introduced into an electron-accepting environment, which in this case is graphene.”
The Rice lab hopes to optimize the connection between the nanoparticles and graphene or another substrate, preferentially a semiconductor that will allow them to trap hot electrons.
“But this experiment wasn’t about making a specific device,” Link said. “It was about measuring the transfer step. Of course, now we’re thinking about designing systems to separate the charge longer, as the electrons transferred quickly back to the gold nanorods. We want to put these hot electrons to work for devices like photodetectors or as catalysts where these electrons can do chemistry.
“It would be fascinating if we could use this process as a source of hot electrons for catalysis and also as an analytical tool for observing such plasmon-enabled reactions. That’s the big picture.”
The paper’s co-authors are Rice graduate students Lin-Yung Wang, Lulu Ma and Jana Olson; former postdoctoral researchers Ying Fang and Zheng Liu; senior Ge You; research scientist Wei-Shun Chang and Pulickel Ajayan, the Benjamin M. and Mary Greenwood Anderson Professor in Mechanical Engineering and Materials Science and of chemistry and chair of Rice’s Department of Materials Science and NanoEngineering. Link is an associate professor of chemistry and of electrical and computer engineering.
The Robert A. Welch Foundation, the National Science Foundation, the Army Research Office and the American Chemical Society Petroleum Research Fund supported the research.