Treating cadmium-telluride (CdTe) solar cell materials with cadmium-chloride improves their efficiency, but researchers have not fully understood why. Now, an atomic-scale examination of the thin-film solar cells led by the Department of Energy's Oak Ridge National Laboratory has answered this decades-long debate about the materials' photovoltaic efficiency increase after treatment.
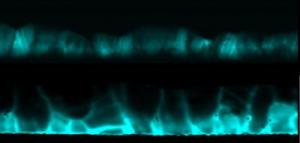 Cross-sectional electron beam-induced current maps show the difference in cadmium telluride solar cells before (pictured above) and after (below) cadmium chloride treatment. The increased brightness after treatment indicates higher current collection at the grain boundaries. Credit: ORNL
Cross-sectional electron beam-induced current maps show the difference in cadmium telluride solar cells before (pictured above) and after (below) cadmium chloride treatment. The increased brightness after treatment indicates higher current collection at the grain boundaries. Credit: ORNL
A research team from ORNL, the University of Toledo and DOE's National Renewable Energy Laboratory used electron microscopy and computational simulations to explore the physical origins of the unexplained treatment process. The results are published in Physical Review Letters (PRL).
Thin-film CdTe solar cells are considered a potential rival to silicon-based photovoltaic systems because of their theoretically low cost per power output and ease of fabrication. Their comparatively low historical efficiency in converting sunlight into energy, however, has limited the technology's widespread use, especially for home systems.
Research in the 1980s showed that treating CdTe thin films with cadmium-chloride significantly raises the cell's efficiency, but scientists have been unable to determine the underlying causes. ORNL's Chen Li, first author on the PRL study, explains that the answer lay in investigating the material at an atomic level.
"We knew that chlorine was responsible for this magical effect, but we needed to find out where it went in the material's structure," Li said. "Only by understanding the structure can we understand what's wrong in this solar cell -- why the efficiency is not high enough, and how can we push it further."
By comparing the solar cells before and after chlorine treatment, the researchers realized that atom-scale grain boundaries were implicated in the enhanced performance. Grain boundaries are tiny defects that that normally act as roadblocks to efficiency, because they inhibit carrier collection which greatly reduces the solar cell power.
Using state of the art electron microscopy techniques to study the thin films' structure and chemical composition after treatment, the researchers found that chlorine atoms replaced tellurium atoms within the grain boundaries. This atomic substitution creates local electric fields at the grain boundaries that boost the material's photovoltaic performance instead of damaging it.
The research team's finding, in addition to providing a long-awaited explanation, could be used to guide engineering of higher-efficiency CdTe solar cells. Controlling the grain boundary structure, says Li, is a new direction that could help raise the cell efficiencies closer to the theoretical maximum of 32 percent light-to-energy conversion. Currently, the record CdTe cell efficiency is only 20.4 percent.
"We think that if all the grain boundaries in a thin film material could be aligned in same direction, it could improve cell efficiency even further," Li said.
The team's research appears as "Grain-Boundary-Enhanced Carrier Collection in CdTe Solar Cells." Coauthors are ORNL's Chen Li, Jonathan Poplawsky, Mark Oxley and Andrew Lupini; University of Toledo's Yelong Wu, Naba Paudel, Wanjian Yin and Yanfa Yan; University of Tennessee's Stephen Pennycook; University of Manchester's Sarah Haigh; University of Oxford's Timothy Pennycook; and NREL's Mowafak Al-Jassim. Li and Oxley hold joint appointments at Vanderbilt University.