May 23 2014
Rice University researchers see nanodiamonds created in coal fade away in seconds
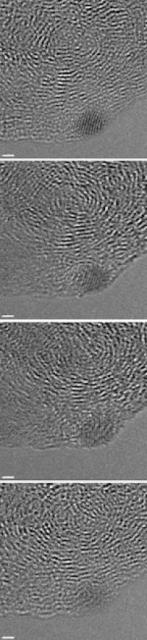 A series of images shows a small nanodiamond (the dark spot in the lower right corner) reverting to anthracite. Rice University scientists saw nanodiamonds form in hydrogenated coal when hit by the electron beam used in high-resolution transmission electron microscopes. But smaller diamonds like this one degraded with subsequent images. The scale bar is 1 nanometer. (Credit: Billups Lab/Rice University)
A series of images shows a small nanodiamond (the dark spot in the lower right corner) reverting to anthracite. Rice University scientists saw nanodiamonds form in hydrogenated coal when hit by the electron beam used in high-resolution transmission electron microscopes. But smaller diamonds like this one degraded with subsequent images. The scale bar is 1 nanometer. (Credit: Billups Lab/Rice University)
Images taken by Rice University scientists show that some diamonds are not forever.
The Rice researchers behind a new study that explains the creation of nanodiamonds in treated coal also show that some microscopic diamonds only last seconds before fading back into less-structured forms of carbon under the impact of an electron beam.
The research by Rice chemist Ed Billups and his colleagues appears in the American Chemical Society's Journal of Physical Chemistry Letters.
Billups and Yanqiu Sun, a former postdoctoral researcher in his lab, witnessed the interesting effect while working on ways to chemically reduce carbon from anthracite coal and make it soluble. First they noticed nanodiamonds forming amid the amorphous, hydrogen-infused layers of graphite.
It happened, they discovered, when they took close-ups of the coal with an electron microscope, which fires an electron beam at the point of interest. Unexpectedly, the energy input congealed clusters of hydrogenated carbon atoms, some of which took on the lattice-like structure of nanodiamonds.
"The beam is very powerful," Billups said. "To knock hydrogen atoms off of something takes a tremendous amount of energy."
Even without the kind of pressure needed to make macroscale diamonds, the energy knocked loose hydrogen atoms to prompt a chain reaction between layers of graphite in the coal that resulted in diamonds between 2 and 10 nanometers wide.
But the most "nano" of the nanodiamonds were seen to fade away under the power of the electron beam in a succession of images taken over 30 seconds.
"The small diamonds are not stable and they revert to the starting material, the anthracite," Billups said.
Billups turned to Rice theoretical physicist Boris Yakobson and his colleagues at the Technological Institute for Superhard and Novel Carbon Materials in Moscow to explain what the chemists saw. Yakobson, Pavel Sorokin and Alexander Kvashnin had already come up with a chart -- called a phase diagram -- that demonstrated how thin diamond films might be made without massive pressure.
They used similar calculations to show how nanodiamonds could form in treated anthracite and subbituminous coal. In this case, the electron microscope's beam knocks hydrogen atoms loose from carbon layers. Then the dangling bonds compensate by connecting to an adjacent carbon layer, which is prompted to connect to the next layer. The reaction zips the atoms into a matrix characteristic of diamond until pressure forces the process to halt.
Natural, macroscale diamonds require extreme pressures and temperatures to form, but the phase diagram should be reconsidered for nanodiamonds, the researchers said.
"There is a window of stability for diamonds within the range of 19-52 angstroms (tenths of a nanometer), beyond which graphite is more stable," Billups said. Stable nanodiamonds up to 20 nanometers in size can be formed in hydrogenated anthracite, they found, though the smallest nanodiamonds were unstable under continued electron-beam radiation.
Billups noted subsequent electron-beam experiments with pristine anthracite formed no diamonds, while tests with less-robust infusions of hydrogen led to regions with "onion-like fringes" of graphitic carbon, but no fully formed diamonds. Both experiments lent support to the need for sufficient hydrogen to form nanodiamonds.
Kvashnin is a former visiting student at Rice and a graduate student at the Moscow Institute of Physics and Technology (MIPT). Sorokin holds appointments at MIPT and the National University of Science and Technology, Moscow. Yakobson is Rice's Karl F. Hasselmann Professor of Mechanical Engineering and Materials Science, a professor of chemistry and a member of the Richard E. Smalley Institute for Nanoscale Science and Technology. Billups is a professor of chemistry at Rice.