This gift from science just keeps on giving. Measurements taken at the National Institute of Standards and Technology (NIST) show why a material already known to be good at separating components of natural gas also can do something trickier: help convert one chemical to another, a process called catalysis.
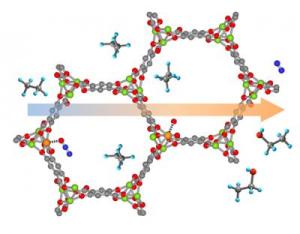 This iron-based metal-organic framework (large structure) can catalyze a reaction that transforms ethane (gray and light blue molecules) into pure ethanol (light blue, red and gray). Scientists think the framework could help reveal ways to mimic other biological functions. Credit: NIST
This iron-based metal-organic framework (large structure) can catalyze a reaction that transforms ethane (gray and light blue molecules) into pure ethanol (light blue, red and gray). Scientists think the framework could help reveal ways to mimic other biological functions. Credit: NIST
The discovery is a rare example of a laboratory-made material easily performing a task that biology usually requires a complex series of steps to accomplish.
The material is a metal-organic framework (MOF), one of a class of substances whose porosity, high surface area and tunable properties make them promising for applications such as gas storage and drug delivery. This particular iron-based MOF, which the research team refers to as Fe-MOF-74,was built in the lab of Jeffrey Long, a professor of chemistry at the University of California Berkeley, who also has patented it.*
Having learned two years ago** that Fe-MOF-74 could effectively separate closely related components of natural gas from one another, this time Long's collaborators at the NIST Center for Neutron Research (NCNR) looked at its power to catalyze reactions—that is, accelerate or enable the chemical reaction of two other materials. In this case, they turned ethane,a component of natural gas, into ethanol, a component of vodka. The research team knew that the iron in the MOF could change from possessing one number of electrons to another, which raised interesting questions.
"One of the big long-term goals of biochemistry is to build things with specific functions from the ground up," says the NCNR's Craig Brown. "It's hard to simply make things like nature does, because she often converts one material into another in a fiendishly complex way. But with a MOF that can mimic nature's effect, we might be able to make the same thing, but right in the lab and far more easily."
While the MOF was great at catalyzing the reaction, the team wasn't sure why. The search for understanding led to two (fairly technical)discoveries at the NCNR: the importance of the MOF's iron for catalysis, and the reason the oxidizer worked so well.
Iron being able to change its number of electrons is the key to creating a high-yield catalytic process. When the team substituted magnesium for 10 percent of the iron in the MOF, the reaction produced 40 percent less ethanol than before. The NCNR's neutron diffractometer helped clarify why, and they also showed that the oxidizer—nitrous oxide, a lopsided molecule with oxygen at one end and two nitrogen atoms at the other—must connect its oxygen end to the iron in the MOF for catalysis to occur.
Brown says exploring the catalytic behavior in this material may reveal other ways to use MOFs to mimic what biology can do. "We hope to get more insights into the reactivity of this material and possibly the design, synthesis and catalytic activities of other MOFs," he says.