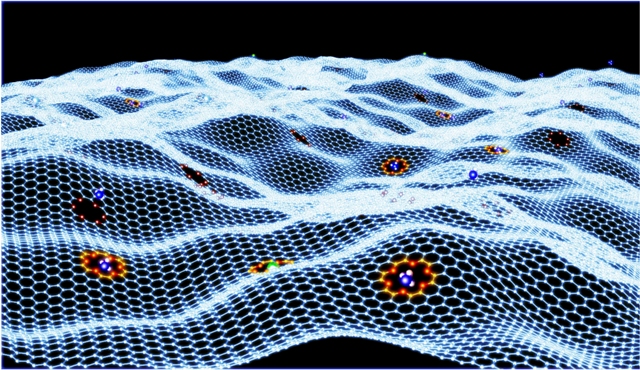
 This sheet of graphene contains an array of crown ethers that can strongly bind select guest ions or molecules. Image credit: Oak Ridge National LaboratoryThis sheet of graphene contains an array of crown ethers that can strongly bind select guest ions or molecules. Image credit: Oak Ridge National Laboratory
This sheet of graphene contains an array of crown ethers that can strongly bind select guest ions or molecules. Image credit: Oak Ridge National LaboratoryThis sheet of graphene contains an array of crown ethers that can strongly bind select guest ions or molecules. Image credit: Oak Ridge National Laboratory
A research team led by the Department of Energy’s Oak Ridge National Laboratory have found a technique to drastically increase the binding strength and selectivity of crown ethers.
The researchers incorporated the crown ethers into a strong graphene framework. Graphene is a one-atom thick, ultra-strong carbon, which was the subject of the subject of the 2010 Nobel Prize in physics.
Ethers are ordinary organic molecules in which an oxygen atom links two carbon atoms and these are chemical building blocks of normal products such as many propellants, solvents, pharmaceuticals and cosmetics. When these are connected together in the form of large molecular rings, they become very significant in the scientific field, forming crown ether molecules, whose development resulted in the 1987 Nobel Prize in chemistry.
These crown-shaped rings hold significance as the initial prototype in host–guest chemistry, a field wherein molecules and “guest” ions can be trapped in a “host” molecule’s cavity. This feature permits chemists to organize a cluster of separately weak bonding reactions such as the bond between a metal ion and an ether oxygen atom, to attain selective, strong binding. This beneficial characteristic also known as “molecular recognition,” is used for sensing, catalysis and separations.
“We’re the first ones to see crown ethers in graphene,” said Matthew Chisholm, who leads the Scanning Transmission Electron Microscopy Group in ORNL’s Materials Science and Technology Division and focuses on material characterization. “Our calculations based on these observations indicate unprecedented selectivity and binding strength.”
Graphene is a stiff sheet because its atoms are formed in a honeycomb structure, including crown ethers into the sheet has forced the ether rings to lie flat. The result is rigid holes optimizing selectivity for atom sizes that rightly fit ring cavities.
Also, restricting the crowns into 2D causes their oxygen dipoles to be inward-facing towards the cavity centres optimizing the electrostatic potential for binding atoms. For instance, the binding strength between crown ether and a potassium atom on graphene in its restricted, rigid state is thrice more than in a unrestricted structure.
The findings have been published in the Nov. 13 issue of Nature Communications and may be the beginning of new supremacy of crown ethers in a range of applications. The specific and robust electrostatic binding may find applications in chemical separations, sensors, nuclear waste cleanup, metal extraction from ores, recycling and purification of rare-earth elements, biotechnology, water purification, medicine, catalysis, energy production in durable lithium-ion batteries and data storage.
The shape and size of the cavity created within a crown ether molecule results in selectivity for complementary ions and small molecules which fit in like a lock and key. There are varying sizes for crown ethers so that ions of varying diameters can be accommodated. In a crown ether, the electric dipole moments of the C–O–C ether groups while structured around a trapped guest metal ion offering a large electrostatic potential for ion binding in the ring cavity. The host can move the guest to areas it cannot go like through cell membranes. Crown ethers are significant for science and technology as only the guest ion can be transported thus.
For almost five decades, scientists have researched the concerted electrostatic binding of crown ether hosts to their ionic guests. Since the molecular recognition characteristics of crown ethers imitate the selective molecular transport characteristics of biological proteins, a new insight into pharmaceutical function has become feasible with interesting medicinal applications.
Host-guest chemistry can be deployed in industrial technology at a small scale for trace ion analysis in aqueous streams at the large scale for removing contaminants such as radioactive cesium from wastes. As crown ethers are selective, they are presently used for metal separations and have already assisted in cleaning up millions of gallons of legacy nuclear waste.
There is however a challenge, which has hindered crown ethers from reaching their full potential in this as well as other applications. Conventional crown ethers are very flexible. They can continuously twist and untwist millions of times each second in solution. Due to their flexibility, the shape and size of a crown ether molecule’ can be adjusted to house varying shapes and sizes of guest ions, restricting the selectivity of the crown ether.
Despite this flexibility, crown ethers cannot take on a suitable shape to bind guest ions restricting their binding strength. Their oxygen atoms are formed in a 3D zigzag manner wherein the C-O-C dipoles do not directly point at the guest ion causing far weaker binding than is normally desired.
This new flattened rigid state of the graphene crown ether implies the flexibility is absent. “Their perfect rigidity is something we almost never see in molecular systems, especially among the traditional crown ethers,” said Bruce Moyer, leader of ORNL’s Chemical Separations Group.
He went on to say: “The oxygens are held in place. There is no way graphene is going to twist. Traditional crown ethers have dipoles that do not point directly at the metal, but the dipoles of the crown ethers in graphene point directly at the guest ion. Graphene thus gives you both enhanced selectivity and enhanced binding for metal ions that fit the crown ether cavity.”
Added Moyer, “Such selectively enhanced binding allows you to do much more challenging separations in principle.” One such application may be mining lithium from sea water, where it is available in low concentrations. Lithium is an element required in electric vehicle batteries among other applications.
These kinds of industrial applications would need ramping up graphene crown ether production. Initial research may require moles, which is a crown ether quantity equal to the number of atoms in 12g of Carbon-12.
“If we have a mole of holes, that’s enough to do bulk chemistry,” Moyer said. “Now we’ve got to figure out how to make a mole of holes.”
ORNL researchers made use of a chemical approach for graphene production. Nidia Gallego and Cristian Contescu began with graphite, oxidized it to form graphene oxide and later reduced that to form graphene.
Since reduction does not get rid of all oxygen the oxygen which remains must have a strong bonding to carbon atoms. When carbon and oxygen alternate around the hole rim in graphene crown ethers are formed in the rigid material.
Chisholm and Junjie Guo, a former postdoctoral researcher at ORNL, made use of electron energy loss spectroscopy and scanning transmission electron microscopy to reveal local composition, atomic position and local electronic properties in the oxidized graphene.
Moyer who shared their research on crown ethers, said he “can hardly wait till we can demonstrate the unprecedented selectivity of the graphene crown ethers in extracting metal ions from solution.”
Sokrates Pantelides with joint appointments at Vanderbilt and ORNL, and Jaekwang Lee from ORNL at Vanderbilt University during the study made use of the VASP program on a supercomputer at the National Energy Research Scientific Computing Center (a DOE Office of Science User Facility at Lawrence Berkeley National Laboratory) to perform theory calculations based on density of graphene sheets functionalized by crown ethers. The computations showed the binding characteristics of planar crown ethers.
The team will study the behaviour of rigid crown ethers. “We’re starting from ground zero,” Chisholm said. “We have seen these crown ether structures in graphene oxide, and now we have to show that they can be made and used.”