Jan 19 2015
Scientists at the Mechanobiology Institute (MBI) at the National University of Singapore (NUS) have discovered the molecular mechanisms responsible for the formation of the adherens junction at the nanoscale level. This research is published in Developmental Cell (Wu et al., Actin-delimited adhesion-independent clustering of E-cadherin forms the nanoscale building blocks of adherens junctions, Developmental Cell, 16 Jan 2015, doi.org/10.1016/j.devcel.2014.12.003).
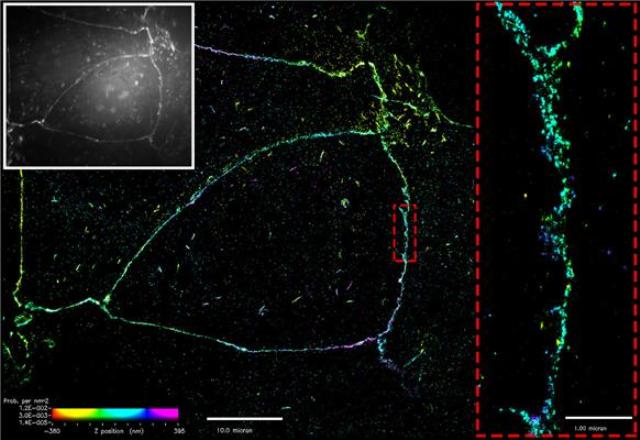 Figure: Superresolution imaging of E-cadherin at the cell membrane. Conventional microscopy (white box) shows E-cadherin as a belt along the cell membrane. Superresolution imaging reveals that E-cadherin assembles as distinct, punctate clusters. Detailed imaging at the nanoscale level demonstrates that these clusters do not merge (dotted red box).
Figure: Superresolution imaging of E-cadherin at the cell membrane. Conventional microscopy (white box) shows E-cadherin as a belt along the cell membrane. Superresolution imaging reveals that E-cadherin assembles as distinct, punctate clusters. Detailed imaging at the nanoscale level demonstrates that these clusters do not merge (dotted red box).
How are cell-cell adhesions initiated?
Although the cells that make up our body are functional units by themselves, they need to interact with each other and their environment to fulfill all their functions. Cells stick to one another as well as to their substrate through physical contacts called cell adhesions. Apart from serving as physical connections that enable cells to form tissues, cell adhesions also allow the cells to sense, signal, and respond to physical or chemical changes in the environment, as well as interact with neighbouring cells.
This is, at least in part, due to the structure of adhesion sites, or cell-cell junctions, which extend through the cell surface into the cell’s interior. At cell-cell junctions, adhesion receptors at the cell surface are linked via adaptor proteins to the cytoskeleton, a structural scaffold inside the cell composed of filamentous proteins like actin. Epithelial cadherin (E-cadherin) is a major adhesion receptor protein which forms a prominent cell-cell adhesion complex called the adherens junction.
Traditionally it was thought that clusters of E-cadherin merge to form a thick belt along the cell membrane between adjacent cells. The binding of individual E-cadherin proteins was thought to drive adhesion, with clusters formed in an adhesion-dependent manner, before merging and becoming uniformly distributed over time. This has long been the prevalent notion, based on conventional microscopy, which is limited in its ability to clearly visualize structures as small as the adherens junction or E-cadherin cluster.
However, recent findings by MBI researchers disprove this notion. Using a combination of an advanced imaging technique called superresolution microscopy along with quantitative analysis and mutational studies, MBI Principal Investigators Associate Professor Ronen Zaidel-Bar and Associate Professor Pakorn Kanchanawong and graduate student Yao Wu show that cell-cell adhesions are initiated by small clusters of about five E-cadherin molecules. Superresolution imaging allowed the nanoscale architecture of the adherens junction to be observed, and distinct, evenly sized E-cadherin clusters were monitored both in the incipient and mature cell-cell adhesions. The precursor E-cadherin cluster forms independently of adhesion, even when mutations prevent E-cadherin interactions, indicating that their formation relies on an alternative mechanism.
As more E-cadherin molecules were recruited from neighbouring cells, the clusters became denser especially at their core. However, E-cadherin clusters never increased in size or merged to form the hypothesized belt. Instead, the actin cytoskeleton was seen to fence E-cadherin clusters, thereby preventing them from merging.
These newly identified steps of adherens junction assembly, organization and maintenance advance our understanding of how adherens junctions adapt to dynamic changes in the behaviour of epithelial cells. Regulating essential functions such as cell shape, movement and rearrangement is vital for maintaining epithelium integrity, and is also important for tissue repair in wound healing and disease.