Jul 6 2015
Researchers from Georgia Institute of Technology and the University of Wisconsin-Madison have developed a new catalyst that could reduce the global reliance on limited, expensive and highly reactive metal, platinum. Platinum is widely used in the energy and chemical industries.
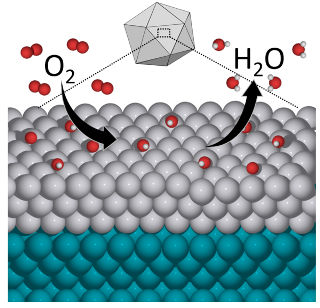 A chemical diagram shows the oxygen reduction reaction, a key chemical reaction in many energy and chemical processes, taking place with an icosahedral particle made of palladium and platinum. (Credit: Mavrikakis group, UW-Madison) capabilities
A chemical diagram shows the oxygen reduction reaction, a key chemical reaction in many energy and chemical processes, taking place with an icosahedral particle made of palladium and platinum. (Credit: Mavrikakis group, UW-Madison) capabilities
The innovative catalyst integrates platinum with palladium, which happens to be a less costly metal. This technique effectively brings down the need for platinum, and at the same time proves to be much more catalytically active compared to using pure platinum in key chemical processes such as oxygen reduction reaction. Such processes are integral in fuel cell energy applications. In addition, the combination of platinum and palladium proves more long-lasting, thus providing more reactivity with minimum amount of material. The study, which has been published in the journal Nature Communications, also provides an advanced way to fabricate a greater number of novel catalysts by simply changing the materials on the atomic scale. Such catalysts, if developed, can be used on a wide variety of applications.
The latest discovery was actually initiated in 2014, when Georgia Tech scientists fabricated nanoparticles that mostly included palladium, with just a slight amount of platinum applied to the surface. It was previously discovered that these nanoparticles displayed much more activity when compared to pure platinum. This was determined by dividing the energy generated by the oxygen reduction reaction by the mass of platinum used. However, to better understand the chemistry driving this obvious benefit, the Georgia Tech team collaborated with the UW-Madison team of Professor Manos Mavrikakis, postdoctoral researcher Jeffrey Herron, and graduate student Luke Roling. Professor Mavrikakis is the Paul A. Elfers Professor and Vilas Distinguished Achievement Professor of Chemical and Biological Engineering.
Roling said that the high performance of the novel catalyst initially appeared to be counterintuitive from a chemist's standpoint. However, the Mavrikakis group leveraged its strengths in computational and modeling analysis and discovered that the benefit lies in the icosahedral structure of the nanoparticle. In the future, scientists in search of innovative catalysts can work on identical structures and find much more reactive materials.
The results justify years of study of the hypothetical as well as the synthesis sides of catalysis, focusing on how the reactivity of a specific substance changes based on whether it is structured as an octahedron, an icosahedron, or any other shape.
"This is speaking to the precise arrangement of atoms on the surface of a nanoparticle. That can make an enormous difference in how fast the reaction takes place. Theory has been instrumental for about 10 years now to demonstrate the importance of being able to tailor-make specific facets of the same material," said Mavrikakis.
"The goal here is to try to minimize the amount of platinum that you use, and eventually find a complete replacement of platinum. If we can move away from platinum, many of these applications have the potential to become more robust financially," he added.
The study could make all kinds of catalysis-driven processes cost-effective and more efficient, as the rapid technological advancements for the synthesis of new and customized materials align with quantum mechanics.
The Department of Energy and the Vilas Distinguished Achievement Professorship supported the study.