Aug 10 2015
Hole-filled crystals called MOFs could one day serve as high-tech sponges, sopping up spilled oil, greenhouse gases and other chemicals.
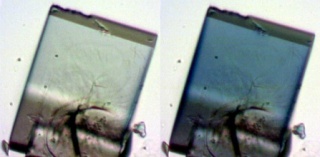 UBMOF-2, a crystalline material, goes from clear to blue when it’s exposed to ultraviolent light. Heating the material up brings it back to its original hue. As the material shifts between colors, its structure is also changing — a property that could enable it to act as a sponge that traps materials inside shape-shifting pores. Credit: Ian Walton
UBMOF-2, a crystalline material, goes from clear to blue when it’s exposed to ultraviolent light. Heating the material up brings it back to its original hue. As the material shifts between colors, its structure is also changing — a property that could enable it to act as a sponge that traps materials inside shape-shifting pores. Credit: Ian Walton
But there’s a problem: Many MOFs — metal-organic frameworks — lose their sponging capabilities over time.
To overcome this hurdle, University at Buffalo chemist Jason Benedict is studying what causes one particularly intriguing type of MOF to fail. He has created light-activated versions of the crystal sponges in his lab, and hopes his work in understanding why they break down will provide insight into how scientists can extend the minute contraptions’ lives.
“These sponges are photoswitchable, which means they’re activated by light,” explains Benedict, an assistant professor of chemistry in UB’s College of Arts and Sciences. “But what happens over time is that the material stops responding to light, so you turn it off and then you can’t turn it on again. We want to find out exactly why this happens. It’s a somewhat tricky thing to study.”
The research is funded by a new $600,000 National Science Foundation (NSF) CAREER award, one of the organization’s most prestigious recognitions for junior investigators.
Saving the UBMOF
Benedict’s study focuses on a series of MOFs his lab created, the UBMOFs. (MOFs are typically named for the institution where they are first synthesized, Benedict says.)
The UBMOFs work by changing their geometry when they’re hit by ultraviolet light.
This causes the shape of individual pores inside the MOFs to morph, which matters because it’s one way to trap materials inside the crystals.
You could, in theory, soak up a chemical and then alter the shape of the pores so that the captured compound can no longer escape. Then, to turn the sponge off and release the contents, you’d expose the MOF to heat, which causes it to return to its original shape.
Why this on-off function stops working over time is the mystery that Benedict and his team are investigating.
“The ability to turn it on and off slowly decays,” Benedict says. “It could be that the chemicals in the crystal are reacting with the environment in a way that degrades the chemistry. It could also be that the compound takes on an unfavorable geometry that doesn’t respond to light or heat in the way that it originally did.”
To understand why the MOFs begin to fail, Benedict will conduct experiments that elucidate how, exactly, the crystals’ structure and chemistry changes in response to light and heat.
The sponges of the future
Benedict is a member of UB’s New York State Center of Excellence in Materials Informatics, which advances the study and development of new materials that could improve life for future generations.
His new NSF CAREER award funding is for basic science: The research on MOFs is still in its early stages, and his team has not yet used them as sponges.
His goal, however, is to find a way to extend the crystals’ lives so that his team can begin sponging experiments that utilize MOFs to trap and release chemicals.
The crystals change color — going from clear to red, or clear to blue — when they’re hit by ultraviolet light, which suggests that their electronic properties are shifting, a characteristic that could also facilitate sponging by attracting different types of chemical compounds into the shape-shifting pores.