 (From left) Peidong Yang, Christopher Chang and Michelle Chang led the development of an artificial photosynthesis system that can convert CO2 into valuable chemical products using only water and sunlight. (Photo by Roy Kaltschmidt)
(From left) Peidong Yang, Christopher Chang and Michelle Chang led the development of an artificial photosynthesis system that can convert CO2 into valuable chemical products using only water and sunlight. (Photo by Roy Kaltschmidt)
Berkeley Lab researchers, who are involved in the development of a bioinorganic hybrid method for artificial photosynthesis, have accomplished another breakthrough.
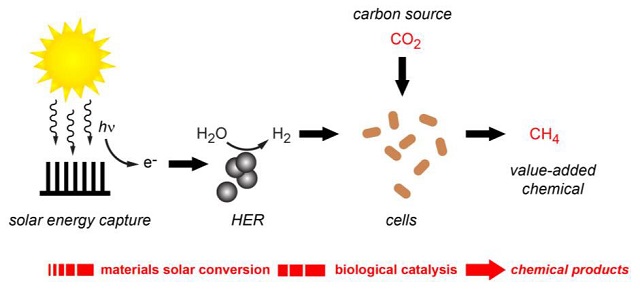
The researchers have created a hybrid system capable of producing renewable molecular hydrogen and utilizing it to convert carbon dioxide into methane, which is a key element of natural gas. Having generated quite a buzz with their hybrid system of semiconducting nanowires and bacteria that used electrons to synthesize carbon dioxide into acetate, the team has now developed a hybrid system that produces renewable molecular hydrogen and uses it to synthesize carbon dioxide into methane, the primary constituent of natural gas.
This study represents another key breakthrough in solar-to-chemical energy conversion efficiency and artificial photosynthesis. By generating renewable hydrogen and feeding it to microbes for the production of methane, we can now expect an electrical-to-chemical efficiency of better than 50 percent and a solar-to-chemical energy conversion efficiency of 10-percent if our system is coupled with state-of-art solar panel and electrolyzer.
Peidong Yang, a chemist with Berkeley Lab’s Materials Sciences Division
Yang is one of three corresponding authors of a paper describing this research in the Proceedings of the National Academy of Sciences (PNAS). He holds appointments with UC Berkeley and the Kavli Energy NanoScience Institute (Kavli-ENSI) at Berkeley. The other corresponding authors are Michelle Chang and Christopher Chang, and they hold joint appointments with Berkeley Lab and UC Berkeley. Additionally Chris Chang is also a Howard Hughes Medical Institute (HHMI) investigator. The paper is titled “A hybrid bioinorganic approach to solar-to-chemical conversion.”
Photosynthesis can be defined as the process wherein solar energy is used by nature to synthesize carbohydrates from water and carbon dioxide. Carbohydrates are biomolecules capable of storing the chemical energy utilized by live cells.
The first hybrid artificial photosynthesis system devised by the Berkeley Lab researchers consisted of a range of silicon and titanium oxide nanowires, which gathered solar energy and supplied electrons to microbes. The microbes then used the electrons for carbon dioxide reduction into a number of value-added chemical products.
In the latest hybrid system, the solar energy is used to separate the water molecule into molecular hydrogen and oxygen. The hydrogen is then transferred to the microbes, which utilize it for carbon dioxide reduction into methane.
In our latest work, we’ve demonstrated two key advances. First, our use of renewable hydrogen for carbon dioxide fixation opens up the possibility of using hydrogen that comes from any sustainable energy source, including wind, hydrothermal and nuclear. Second, having demonstrated one promising organism for using renewable hydrogen, we can now, through synthetic biology, expand to other organisms and other value-added chemical products.
Chris Chang
Both the hybrid systems follow the same concept that involves populating the membrane of semiconductor nanowires capable of harnessing solar energy with bacterium, which can generate a specific carbon-based chemical using the solar energy.
The membrane in the current research is composed of titanium dioxide photoanodes and indium phosphide photocathodes, while in the previous study, the membrane used was Sporomusa ovata, which is an anaerobic bacterium capable of easily absorbing electrons from the nearby environment for carbon dioxide reduction.
For the current research, the team filled the membrane with Methanosarcina barkeri, an anaerobic archaeon capable of reducing carbon dioxide using hydrogen instead of electrons.
Using hydrogen as the energy carrier rather than electrons makes for a much more efficient process as molecular hydrogen, through its chemical bonds, has a much higher density for storing and transporting energy.
Michelle Chang.
The latest membrane used by the team enables solar energy to be absorbed and used to produce hydrogen from water through a hydrogen evolution reaction (HER). Earth-abundant nickel sulfide nanoparticles, which function well under biologically suitable conditions, are used to catalyze the HER. The resultant hydrogen generated during the HER is utilized by the Methanosarcina barkeri archaeons present in the membrane for methane generation.
“We selected methane as an initial target owing to the ease of product separation, the potential for integration into existing infrastructures for the delivery and use of natural gas, and the fact that direct conversion of carbon dioxide to methane with synthetic catalysts has proven to be a formidable challenge,” says Chris Chang. “Since we still get the majority of our methane from natural gas, a fossil fuel, often from fracking, the ability to generate methane from a renewable hydrogen source is another important advance.”
Adds Yang, “While we were inspired by the process of natural photosynthesis and continue to learn from it, by adding nanotechnology to help improve the efficiency of natural systems we are showing that sometimes we can do even better than nature.”
Along with the corresponding authors, the other co-authors of the study included Eva Nichols, Joseph Gallagher, Chong Liu, Yude Su, Joaquin Resasco, Yi Yu and Yujie Sung.
The DOE Office of Science primarily funded this Berkeley research.
References