Sep 26 2015
For scientists to understand a system, they often push it to its limits. In geochemistry, that means putting minerals under extreme conditions and watching how they react.
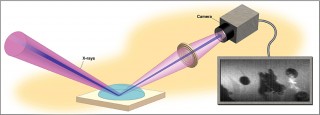 A team of physicists and geochemists at Argonne and Oak Ridge National Laboratory have shown that instead of just passively observing surface reactions of minerals, they can use X-rays to create the conditions by which reactions happen while simultaneously observing them.
A team of physicists and geochemists at Argonne and Oak Ridge National Laboratory have shown that instead of just passively observing surface reactions of minerals, they can use X-rays to create the conditions by which reactions happen while simultaneously observing them.
This can be done in a number of ways, but the approach is usually the same: develop tools necessary to observe reactions in better detail and look at how minerals react when their natural environment is destabilized.
The X-ray Reflection Interfacial Microscope, a new surface microscope at the U.S. Department of Energy's (DOE's) Argonne National Laboratory, has led to a major breakthrough. By using powerful photon beams generated by the Advanced Photon Source (APS), a DOE User Facility located at Argonne, researchers have shown that they can now control the chemical environment and provide nanoscale structural detail while simultaneously imaging the mineral calcite as it is pushed to its extremes.
"There are some very extreme natural environments on our planet," said Argonne's Paul Fenter, Interfacial Processes Group Leader and co-author of the study appearing today in the journal Science. "If you can understand how minerals react at the most extreme conditions, this gives you confidence in our understanding of reactions under less extreme conditions."
A story of collaborative success, the Interfacial Microscope and the techniques that arise from it were enabled by a Partnership User Proposal agreement that gave Nouamane Laanait (a former postdoctoral fellow at Argonne) dedicated time to work on the beamline to develop the instrument in exchange for opening it up to use to all users.
With this tool, a team of physicists and geochemists at Argonne and Oak Ridge National Laboratory have shown that, instead of just passively observing surface reactions of minerals, they can use X-rays to create the conditions by which reactions happen while simultaneously observing them.
Traditionally, researchers study how a mineral grows and dissolves by measuring how much and how fast it dissolves under a flowing solution. But this method does not tell researchers about the process by which the material dissolves. In addition, the act of flowing solution over a material while taking measurements presents certain challenges.
Breaking it apart to put it back together
Our natural world rests in a delicate balance controlled by the movement of nutrients and toxins through waterways. Minerals like calcite grow and dissolve in response to changes in the water composition, which can be characterized by its level of acidity (i.e., the pH). A key feature of this experiment was the use of the X-rays to drive the calcite out of equilibrium while simultaneously observing how it dissolves.
"These reactions are well-known," said Nouamane Laanait, the paper's first author and current Eugene P. Wigner Fellow at Oak Ridge National Laboratory. "They are the same as those that control how calcite dissolves in oceans in response to increased CO2 levels. This work demonstrates that if one has precise control over the beam probe and appropriate modeling of the beam interactions [with the sample], then one can learn a great deal that would be inaccessible otherwise."
To see what happens to the calcite when it is destabilized, researchers used a technique called X-ray reflection interface microscopy (XRIM) at the APS.
Piercing through water solution and reflecting off the calcite's surface like a mirror, focused X-rays changed the water's acidity level, starting a chain of reactions that lowered the pH and caused the calcite to dissolve. Tiny pits, similar to ones observed in previous experiments, began to form with simple round or rectangular shapes. The rate at which these pits formed and grew let researchers know that the X-ray beam was, in fact, controlling the local chemistry as predicted. What they didn't predict came next.
As the X-rays pushed the calcite to more extreme levels of instability, researchers were surprised to see that the dissolving pits became distorted and formed ink splatter-like irregularities, indicating that some parts were dissolving quicker than others. Known as reaction front instabilities, these irregularities had not previously been observed in real time.
"Calcite is well-studied," said Fenter, "and so we have a very good understanding of how it grows and dissolves over a wide range of conditions. That we were able to observe a new mode of dissolution was exciting since it suggests that there is still much to be learned."
This research is detailed in the paper "X-ray–driven reaction front dynamics at calcite-water interfaces," published in Science. This work was led by the Interfacial Processes group within Argonne's Chemical Sciences and Engineering division. Additional co-authors include Sang Soo Lee, Neil C. Sturchio, Erika B. R. Callagon, and Zhan Zhang (APS).
This work was supported by the Geosciences Research Program of the Office of Basic Energy Sciences, U.S. Department of Energy (DOE), at Argonne National Laboratory, the University of Illinois at Chicago, and the University of Delaware. The X-ray data were collected at the Advanced Photon Source (33-ID-D), a DOE Office of Science User Facility at Argonne.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. Department of Energy's Office of Science.
DOE's Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.