Mar 16 2016
Researchers at the Houston Methodist Research Institute have developed a unique drug that effectively removes lung metastases in mice. This latest breakthrough may radically redefine the treatment of metastatic triple negative breast cancer.
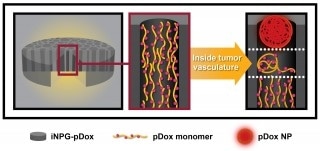 iNPG process (Houston Methodist Research Institute)
iNPG process (Houston Methodist Research Institute)
The results of the study have been published in the early online edition of Nature Biotechnology. The paper illustrates the action of an injectable nanoparticle generator (iNPG), and demonstrates how a complicated technique of transporting a nano-version of a standard chemotherapy drug resulted in positive outcomes in mice models that had triple negative breast cancer, which had metastasized to the lungs.
Most cancer deaths occur due to metastases to the liver and lung organs, however there is no cure. Current cancer drugs do not provide much benefit, as they lack the ability to conquer biological obstacles in the body, and fail to reach the tumor cells in adequate concentrations. This issue has now been resolved by cancer and nanotechnology experts at the Houston Methodist Research Institute, who have developed a special drug that produces nanoparticles within the lung metastases in mice.
In the landmark study, half of the mice treated with the new drug did not show any trace of the metastatic disease after a period of eight months. In humans, this is equivalent to roughly 24 years of extended survival after the occurrence of metastatic breast cancer.
Mauro Ferrari, Ph.D, president and CEO of the Houston Methodist Research Institute, said that the body has its own defense mechanisms. As a result only a part of the injected cancer drug reaches the tumor site, while most of the drug is absorbed by healthy tissues leading to adverse side effects. This is what makes cancer drugs less effective.
The latest treatment strategy allows a sequential passage of the biological obstacles to transport the cancer-killing agent to the tumor site. This active drug is released only within the metastatic disease cell nucleus, preventing the multidrug resistance mechanism exhibited by the tumor cells. This approach does not only destroy the cancer cells, it also provides considerable remedial benefit in the mice, including extended survival in 50% of the animals. This latest result has come after years of research following Ferrari’s work in nanomedicine. Haifa Shen, M.D., Ph.D., and Ferrari are co-senior authors of the paper.
This may sound like science fiction, like we’ve penetrated and destroyed the Death Star, but what we discovered is transformational. We invented a method that actually makes the nanoparticles inside the cancer and releases the drug particles at the site of the cellular nucleus. With this injectable nanoparticle generator, we were able to do what standard chemotherapy drugs, vaccines, radiation, and other nanoparticles have all failed to do.
Mauro Ferrari, Ph.D, President and CEO, Houston Methodist Research Institute
Houston Methodist Research Institute intends to speed up the research work to acquire FDA approval and initiate safety/efficacy trial studies in humans by next year. Consequently, it has devised good manufacturing practices for this active drug.
I would never want to overpromise to the thousands of cancer patients looking for a cure, but the data is astounding. We’re talking about changing the landscape of curing metastatic disease, so it’s no longer a death sentence.
Mauro Ferrari, Ph.D, President and CEO, Houston Methodist Research Institute
The research team at Houston Methodist Research Institute utilized a cancer drug called doxorubicin, which has been used for many years, but this drug causes many negative side effects to the heart and is not effective to treat metastatic cancer diseases. During the experimental study, the team used the injectable nanoparticle generator, which is made up of several components. The doxorubicin drug was embedded into this nanoparticle generator.
Shen, a senior member of the department of nanomedicine at Houston Methodist Research Institute, explained that individual components play a critical and specific role in the process of drug delivery. Nanoporous silicon material is the first component that naturally decomposes in the body, and a polymer made up of different strands containing doxorubicin forms the second component. Once the silicon material is within the cancer cells, it decomposes and releases the strands. As a result of natural thermodynamic forces, the strands twist-up to form nanoparticles, which are then absorbed by the tumor cells. When these nanoparticles are within the tumor cells, the acidic pH close to the nucleus makes the nanoparticles release the drug. This active drug within the nucleus then acts to destroy the cancer cell.
If this research bears out in humans and we see even a fraction of this survival time, we are still talking about dramatically extending life for many years. That’s essentially providing a cure in a patient population that is now being told there is none.
Mauro Ferrari, Ph.D, President and CEO, Houston Methodist Research Institute
Ferrari holds the Ernest Cockrell Jr. Presidential Distinguished Chair and is considered one of the founders of nanomedicine and oncophysics (physics of mass transport within a cancer lesion).
According to the researchers, the active drug could aid cancer physicians to treat lung metastases arising from other origins, including primary lung cancers.
Other researchers who contributed to the paper were Rong Xu, Junhua Mai, Guodong Zhang, Xiaoyong Deng, Suhong Wu, Victor Segura-Ibarra, Haoran Liu, Jianliang Shen, Zhenhua Hu, Yi Huang, Yu Huang, Lingxiao Chen, Eugene Koay, Xuewu Liu, and Elvin Blanco from the Department of Nanomedicine, Houston Methodist Research Institute, Houston, Texas; Joe Ensor from Houston Methodist Cancer Center, Houston, Texas; and Jun Liu from the Department of Pathology and Laboratory Medicine, The University of Texas-Houston Medical School.
The study was funded by grants from National Institute of Health (U54CA143837 and U54CA151668), Department of Defense (W81XWH-09-1-0212 and W81XWH-12-1-0414), and The Cockrell Foundation.