Mar 21 2016
Solar fuels are clean fuels harvested from sunlight, water, and carbon dioxide, and they provide a way of storing solar energy, for instance in hydrogen or hydrocarbons. However efficiency is still a concern for this technology. Kasper Wenderich of the MESA+ Institute for Nanotechnology of the University of Twente has created special nanosized plates with platinum particles on them to speed up the chemical conversion. As part of his PhD thesis, he determined the reason why the reduced effect of these particles is lower than generally expected.
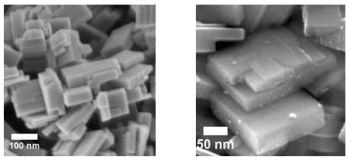 Tungsten oxide nanoplates with the surfaces (facets) on which the reactions take place. Right image: platina particles don't always land on the intended surface.
Tungsten oxide nanoplates with the surfaces (facets) on which the reactions take place. Right image: platina particles don't always land on the intended surface.
Storage is the major challenge when converting solar energy into electric current. The electricity demand is often not in line with the production, as scientists are still exploring ways of storing solar energy in sustainable ‘solar fuels.’ One fuel option is hydrogen, using sunlight and water as a basis. Another option is carbon dioxide conversion, which is considered to be an attractive approach to tackle the greenhouse emission problems.
Photocatalysts are required to handle the chemical reactions involved. The commonly used material is titanium dioxide, but it has its own limitations according to Wenderich. Tungsten oxide is the material of choice for Wenderich due to its ability to adsorb sunlight. Using the yellow material, he created nano plates. On the surfaces or facets of these special crystal structures, the chemical reactions will occur. The release of electrons from the surface by the sunlight leads to the formation of hydrogen, upon adsorption of hydrogen ions at the surface. It is a challenging task to guide the electrons in the right directions. The freed ‘holes’ shift to one of the other plate surfaces and react with the oxygen ions towards oxygen gas.
An extra push is required for tungsten oxide to achieve higher efficiency. It is a general belief that platina nanoparticles are capable of serving as a co-catalyst in reactions involving electrons. The photo deposition technique is used to place these particles on the reactive surfaces. Theoretically, the electrons should move to the correct surface in the presence of platina. However, placing platina on the correct surfaces is a challenging task, according to Wenderich. Platina has the tendency to shift towards the edges rather than the surfaces, slowing down the reaction rather than accelerating it.
This scenario was demonstrated by Wenderich with a solar fuel experiment, where the yield was less than expected. He also explored the mechanisms thoroughly with the help of an atomic force microscope. Wenderich’s thesis helps to understand the optimum distribution of particles and their size. Even though the results were not up to the mark, photo deposition is still considered a better option for generation of solar fuels. Besides tungsten oxide, the nano plates can be used with other materials, paving the way for new research works in the future.
Wenderich’s thesis ‘Photodeposition of platinum nanoparticles on well-defined tungsten oxide’ can be downloaded as a pdf file. He carried out his research work in the Photocatalyst Synthesis group of Professor Guido Mul, which is part of the MESA+ Institute for Nanotechnology of the University of Twente.