May 23 2016
A team of researchers from University of Cincinnati (UC) in Cincinnati, OH have developed a novel microfluidic device, which combines the inertial effect of fluid and microscale vortices generated in microchambers, to achieve simultaneous double sorting of rare target cells and removal of background cells. Sorting and purification of target cells from complex cellular samples is a critical sample preparation step in cell biology research and clinical diagnostics. This task becomes even more challenging for samples containing orders of magnitude larger number of background cells and only a small fraction of target cells, because sorting of such samples not only requires efficient extraction of the target cells but also highly efficient removal of the background cells. The device presented in this work accomplished this challenging task by enabling double sorting functionality that can extract large target cells from background cells in a continuous and automatic fashion. The report appears in a forthcoming issue of the journal TECHNOLOGY.
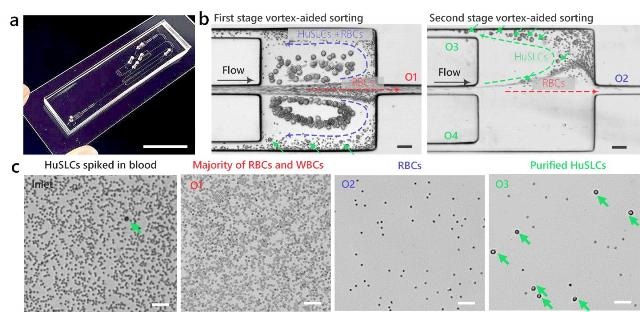 Sorting and purification of human cancer stem-like cells (HuSLCs) from human blood using the novel inertial microfluidic vortex sorter. (a) Photograph of the microfluidic device. (b) Microscopic bright-field images showing the double sorting and purification of HuSCLs from human red blood cells (RBC) using the two microvortex chambers. (c) Microscopic bright-field images illustrating samples before sorting (inlet) and after sorting (outlets O1,O2,O3) indicating successful purification of HuSLCs from outlet 3 (O3). The scale bars are 10 mm in (a) and 50 ?m in (b)-(c). (Credit: TECHNOLOGY)
Sorting and purification of human cancer stem-like cells (HuSLCs) from human blood using the novel inertial microfluidic vortex sorter. (a) Photograph of the microfluidic device. (b) Microscopic bright-field images showing the double sorting and purification of HuSCLs from human red blood cells (RBC) using the two microvortex chambers. (c) Microscopic bright-field images illustrating samples before sorting (inlet) and after sorting (outlets O1,O2,O3) indicating successful purification of HuSLCs from outlet 3 (O3). The scale bars are 10 mm in (a) and 50 ?m in (b)-(c). (Credit: TECHNOLOGY)
"Microfluidics has been an enabling technology in recent two decades. The development in this field led to a large numbers of fascinating tools for a wide range of applications, including molecular biology, cell biology, and clinical diagnostics. Our microfluidic device is able to sort cells label-free, based on their size, continuously and automatically. The unique feature of this device is that it can isolate and extract larger target cells, while eliminating nearly all non-target cells and yielding highly purified cells of interest. This purified cellular sample is beneficial for downstream biomedical research and diagnostics," says Professor Ian Papautsky of the University of Cincinnati and Principal Investigator on the paper.
Although previous microfluidic devices demonstrate sorting of cells with efficiency >95%, it is often insufficient to obtain highly purified target cells if sample contains non-target cells in concentrations multiple orders of magnitude higher than that of the target cells. The microfluidic device in this work introduces an integrated vortex-based inertial microfluidic chip for continuous double sorting and purification of biomicroparticles with high efficiency and purity. The device first uses an inertial effect of fluid in microscale channels to focus fast-flowing cells into highly ordered streaks. Downstream, the first pair of microchambers generates microscale vortices and as cells pass through, the larger target cells are extracted and exit through the two outlets at the corners of the chamber. The smaller background cells elute from the middle outlet. To further remove the remaining background cells and purify large target cells, the two target-cell outlets feed into to a second pair of microchambers to enable the double sorting function that yields highly purified target cell product. To demonstrate the feasibility of sorting of rare cells as well as efficient removal of a large number of background cells, a small number of human cancer stem-like cells (HuSLCs) was spiked into human blood. The device successfully isolated the HuSLCs, while removing >99.97% of the non-target RBCs.
"The double sorting and purification functionality is unique, but this is not the only exciting aspect of this device," says Dr. Xiao Wang, the lead author of the paper. "Cellular samples contain cells of different size. Thus, flexibility of tuning the sorting and extraction cutoffs is critical for maintaining performance. For most of today's microfluidic devices, the sorting cutoff is modified by re-designing and re-fabricating the device. This leads to longer development time, higher cost, and possibly delays in processing of time-sensitive biological samples. In our device, we are able to tune the sorting cutoff diameter by simply changing the input flow rate or by modifying the fluidic resistance without the burden of re-designing and re-fabricating the entire device."
Now, the team at the University of Cincinnati is working further towards optimizing the device to accomplish more challenging cell sorting tasks, such as isolation of the extremely rare circulating tumor cells (CTC) from cancer patients' blood. In addition, they are also working to expand the functionalities of this vortex sorting platform. "Similar to integrating transistors into an integrated circuit, the vortex sorter is a building block that can potentially be integrated into more sophisticated fluidic networks to provide more complex cell sorting functions," says Dr. Xiao Wang. "We hope this integrated vortex sorting platform can ultimately become a versatile and reliable tool for cell sorting applications."