Nov 4 2016
 Hydrogenation (in red) of bilayer graphene via Birch-type reaction begins from the edges.
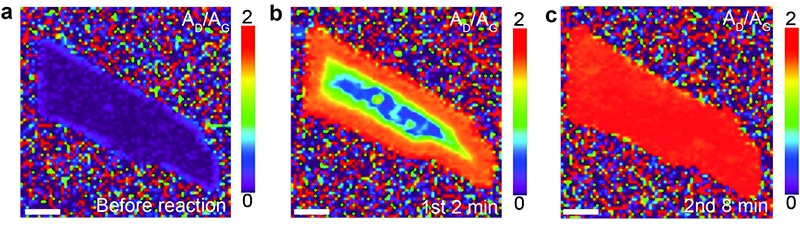
The images show a graphene flake before (a), two minutes (b), and eight minutes (c), after exposure to a solution of lithium and liquid ammonia (Birch-type reaction).
Graphene gets gradually hydrogenated starting from the edges. (Reprinted with permission from Zhang X et al, JACS, Copyright 2016 American Chemical Society)
Hydrogenation (in red) of bilayer graphene via Birch-type reaction begins from the edges.
The images show a graphene flake before (a), two minutes (b), and eight minutes (c), after exposure to a solution of lithium and liquid ammonia (Birch-type reaction).
Graphene gets gradually hydrogenated starting from the edges. (Reprinted with permission from Zhang X et al, JACS, Copyright 2016 American Chemical Society)
The addition of hydrogen to graphene could improve its future applicability in the semiconductor industry, when silicon leaves off. Recently, researchers at the Center for Multidimensional Carbon Materials (CMCM) within the Institute for Basic Science (IBS) gained additional insights into this chemical reaction.
These findings that have been published in Journal of the American Chemical Society enhance the understanding of the basic chemistry of graphene and also bring scientists closer to recognizing new graphene-based materials.
The applications of graphene will increase with a better understanding of how graphene can chemically react with a wide range of chemicals. Graphene is known for its advanced conductivity properties, but it is not possible to directly use it as an alternative to silicon in semiconductor electronics as it lacks a bandgap - its electrons are capable of moving without climbing any energy barrier.
Hydrogenation of graphene opens a bandgap in graphene so that it could be used as a semiconductor component in new devices.
This study, unlike other studies that explain the hydrogenation of bulk materials, deals with hydrogenation of single and few-layers thick graphene. The "Birch-type reaction", which is based on lithium dissolved in ammonia, was used by the IBS scientists in order to introduce hydrogen onto graphene through the development of C-H bonds.
The researchers identified that hydrogenation proceeds rapidly over the whole surface of the single-layer graphene, while it proceeds slowly and from the edges of few-layer graphene. The team further demonstrated that edges or defects are essential for the reaction to take place under the conditions used, because pristine graphene with the edges covered in gold does not go through hydrogenation.
IBS scientists used trilayer and bilayer graphene and identified that the reagents can travel between the layers, and can also equally hydrogenate every single layer. Finally, the scientists discovered that the hydrogenation significantly changed the electric and optical properties of the graphene.
A primary goal of our Center is to undertake fundamental studies about reactions involving carbon materials. By building a deep understanding of the chemistry of single-layer graphene and a few layer graphene, I am confident that many new applications of chemically functionalized graphenes could be possible, in electronics, photonics, optoelectronics, sensors, composites, and other areas.
Rodney Ruoff, CMCM director, and UNIST Distinguished Professor at the Ulsan National Institute of Science and Technology