Feb 23 2017
Now, just months after a Northwestern Engineering and Argonne National Laboratory team discovered the material, another team led by Mark Hersam is already making strides toward understanding its complicated chemistry and realizing its electronic potential.
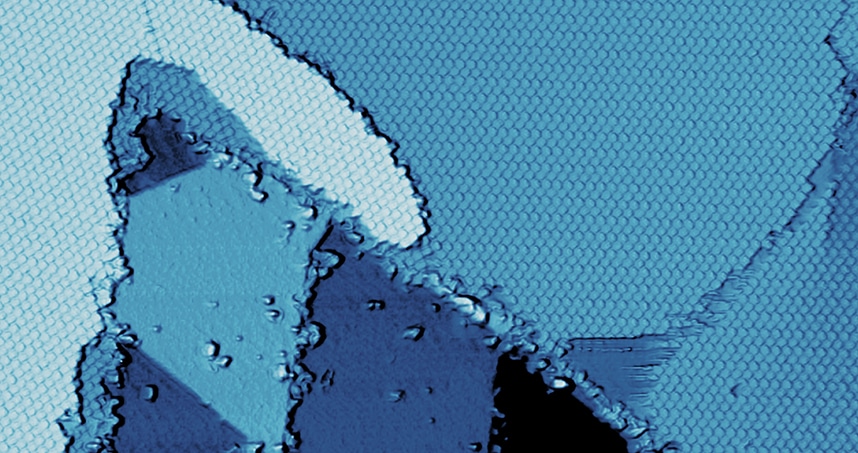 A molecular-resolution image of the borophene-organic material interface. Credit: Mark C. Hersam
A molecular-resolution image of the borophene-organic material interface. Credit: Mark C. Hersam
Created in December 2015, borophene is a two-dimensional, metallic sheet of boron, the element commonly used in fiberglass. Although borophene holds promise for possible applications ranging from electronics to photovoltaics, these applications cannot be achieved until borophene is integrated with other materials. Now Hersam’s team — and a bit of serendipity — have successfully accomplished this integration.
“Integrated circuits are at the heart of all of our computers, tablets, and smartphones,’” said Hersam, Walter P. Murphy Professor of Materials Science and Engineering in Northwestern’s McCormick School of Engineering. “Integration is the key element that has driven advances in electronic technology.”
Supported by the Office for Naval Research and National Science Foundation, the research appeared online on February 22 in the journal Science Advances. Erik Luijten, professor of materials science and engineering at Northwestern Engineering, co-authored the paper. Xiaolong Liu, a student in Northwestern’s Applied Physics Graduate Program, is the paper’s first author.
Because borophene does not appear in nature, scientists must grow it in the laboratory by synthesizing it on a sheet of silver. Hersam’s team deposited an organic material (perylene-3,4,9,10-tetracarboxylic dianhydride) on top of the borophene, in an attempt to integrate the two materials. What happened next was a surprise. The organic material, which is known to self-assemble on essentially any material, instead diffused off the borophene and onto the silver sheet.
The result was a self-assembled monolayer of the organic material directly next to the borophene, forming a nearly perfect interface. Well-controlled interfaces between distinct materials enable integrated devices, including diodes and photovoltaics. Hersam’s surprising technique bypassed the typical challenge to creating a sharp interface — getting materials to touch but not mix.
“This is a nice bit of serendipity because we solved a problem without any additional intervention required,” Hersam said. “Borophene did not exist a year ago. Twelve months later, we’re already forming essentially perfect interfaces.”
Not only does Hersam’s finding set the stage to explore electronic applications for borophene, it also illuminates the new material’s fundamental properties. The next challenge is to move borophene off silver and onto an inert substrate that does not interfere with its electronic properties.
“Borophene is unique in its ability to form abrupt interfaces via self-assembly,” Hersam said. “We’re beginning to understand its chemistry, which will guide our efforts to transfer the material onto appropriate substrates for further integration.”