Mar 23 2017
 Clusters looks just like a multiple-strand necklace. Credit: University of Jyv�skyl�
Clusters looks just like a multiple-strand necklace. Credit: University of Jyv�skyl�
A research study demonstrating the possibility to attain extremely high quality crystals developed from gold nanoparticles has been published in the renowned journal called the Journal of the American Chemical Society by a research team headed by Professor Flavio Maran of the University of Padova (Italy) and Academy Professor Kari Rissanen of the University of Jyväskylä (Finland).
“The research on gold nanoparticles is a field of both fundamental and applied importance” explains Academy Professor Kari Rissanen of the Department of Chemistry at the University of Jyväskylä. X-ray crystallography is considered to be the most powerful method suitable for molecular-structure determination of these nanosystems.
However, obtaining superior quality single crystals ideal for accurate X-ray analysis has indeed been the bottleneck in this challenging research. Recently, this problem was successfully addressed with the help of an electrochemical strategy called electrocrystallization.
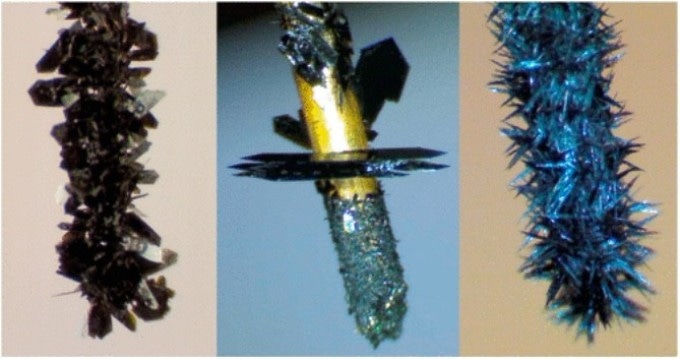
X-ray single crystal diffraction analysis of gold nanoclusters has the basic limitation that single crystals of superior quality are extremely difficult to obtain. Gold nanoclusters are structures comprising of a core developed from dozens gold atoms capped and protected by a single layer of molecules.
An electrochemical method allowing the growth of high-purity crystals in huge quantities and extremely high crystallographic quality has been developed by the team. It is possible to generate dense forests of millimeter-long single crystals directly onto the electrode surface by allowing an extremely small current to pass between two electrodes.
Professor Maran explains that the breakthrough nature of this electrocrystallization method was put into practice by the single crystal X-ray crystallographic determination of the structures of four different nanoclusters, where each nanocluster was made up of 25 gold atoms.
These successful outcomes validated the efficiency of the electrochemical technique and also discovered that one of these clusters crystallizes by developing needles in parallel chains of interconnected gold nanoclusters, resembling a multiple-strand necklace made of gold "pearls" of just one-billionth of a meter.