Dec 19 2017
Visualize a material as lightweight and flexible as foil that turns hard and stiff enough to stop a bullet on impact. In a recently published paper in Nature Nanotechnology, researchers across The City University of New York (CUNY) report a process for making diamene: flexible, layered sheets of graphene that become harder than diamond and impenetrable upon impact on a temporary basis.
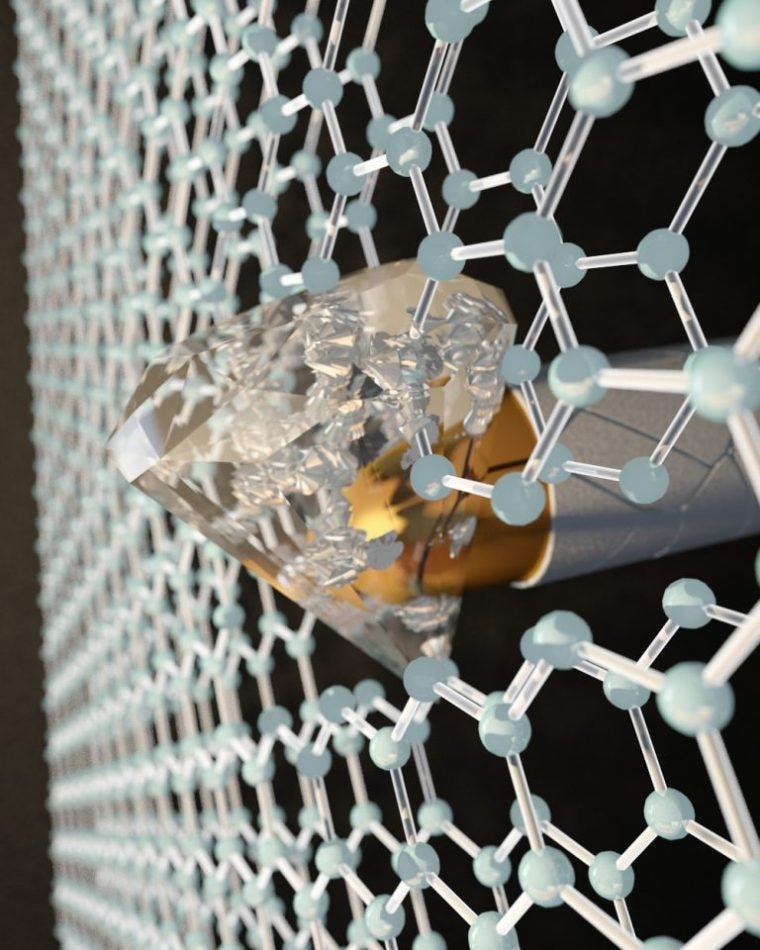 By applying pressure at the nanoscale with an indenter to two layers of graphene, each one-atom thick, CUNY researchers transformed the honeycombed graphene into a diamond-like material at room temperature. Photo credit: Ella Maru Studio
By applying pressure at the nanoscale with an indenter to two layers of graphene, each one-atom thick, CUNY researchers transformed the honeycombed graphene into a diamond-like material at room temperature. Photo credit: Ella Maru Studio
Researchers at the Advanced Science Research Center (ASRC) at the Graduate Center, CUNY, aimed to theorize and analyze how two layers of graphene, each measuring just one-atom in thickness, could be made to convert into a diamond-like material upon impact at room temperature. The team also discovered the moment of conversion caused an unexpected reduction of electric current, signifying diamene could have exciting spintronic and electronic properties. The new findings will probably have applications in creating ultra-light bullet-proof films and wear-resistant protective coatings.
This is the thinnest film with the stiffness and hardness of diamond ever created, previously, when we tested graphite or a single atomic layer of graphene, we would apply pressure and feel a very soft film. But when the graphite film was exactly two-layers thick, all of a sudden we realized that the material under pressure was becoming extremely hard and as stiff, or stiffer, than bulk diamond.
Elisa Riedo, professor of physics at the ASRC and the project’s lead researcher.
Angelo Bongiorno, associate professor of chemistry at CUNY College of Staten Island and part of the research team, formulated the theory for developing diamene. He and his colleagues used atomistic computer simulations to model probable results when pressurizing two honeycomb layers of graphene aligned in diverse configurations. Riedo and other team members then employed an atomic force microscope to apply localized pressure to two-layer graphene on silicon carbide substrates and discovered flawless agreement with the calculations. Experiments and theory both revealed that this graphite-diamond transition does not take place for more than two layers or for one graphene layer.
“Graphite and diamonds are both made entirely of carbon, but the atoms are arranged differently in each material, giving them distinct properties such as hardness, flexibility and electrical conduction,” Bongiorno said. “Our new technique allows us to manipulate graphite so that it can take on the beneficial properties of a diamond under specific conditions.”
According to the paper, the research team’s successful work paves way for possibilities in exploring graphite-to-diamond phase transition in 2D materials. Future research could explore approaches for stabilizing the transition and allow for more applications for the resultant materials.
This research received funding from the BES Office of the Department of Energy and the entire list of authors includes Yang Gao, Tengfei Cao, Filippo Cellini, Claire Berger, Walt Heer, Erio Tosatti, Angelo Bongiorno, and Elisa Riedo.