Feb 6 2018
Although cancer-fighting nanovaccines have demonstrated immense potential, their clinical application has been hindered by complexities in quality control, large-scale manufacturing, and protection.
Biomedical engineers from the National Institute of Biomedical Imaging and Bioengineering (NIBIB) have created an innovative technology that allows nanovaccines to adhere to the albumin protein that naturally exists inside the human body. Then, the albumin protein distributes these nanocomplexes to the lymph nodes, leading to vigorous immune activation against different types of tumors in mouse cancer models. The application of natural albumin as a universal vaccine shuttle is an important move in administering cancer nanovaccine immunotherapy to people.
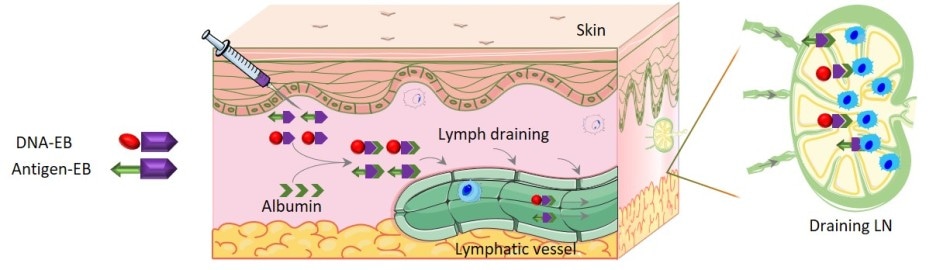 Schematic of self-assembly of the AlbiVax nanovaccine. The tumor-specific antigen and DNA segment were attached to Evans blue (purple arrows). Upon injection, albumin in the blood (green arrows) binds to the Evans blue and brings the complexes into the lymph system where the DNA and antigen interact with the immune cells (blue) in the lymph nodes. The interaction triggers a vigorous immune response against the target tumor. Credit: Zhu, et al.
Schematic of self-assembly of the AlbiVax nanovaccine. The tumor-specific antigen and DNA segment were attached to Evans blue (purple arrows). Upon injection, albumin in the blood (green arrows) binds to the Evans blue and brings the complexes into the lymph system where the DNA and antigen interact with the immune cells (blue) in the lymph nodes. The interaction triggers a vigorous immune response against the target tumor. Credit: Zhu, et al.
So, what’s new?
Nanovaccines that function to form an immune response against a tumor fundamentally comprise of two components: the part that distributes the vaccine to the exact site (the lymph nodes) in which the immune system stimulation occurs; and the part that stimulates the immune cells to enlarge and particularly target the tumor.
We designed a vaccine that binds to a protein called albumin normally found in the body, that also regularly filters through the lymph nodes. Thus, the vaccine essentially hitches a ride with albumin to travel to the lymph nodes, eliminating the need to create a separate delivery vehicle. Given that large-scale manufacturing and long-term safety are the primary hurdles of current nanovaccine technology, our approach offers a detour to accelerate eventual use of nanomedicines in the clinic.” The study has been explained in the December 2017 issue of Nature Communications.
Guizhi Zhu, Ph.D., First Author of the study and Post-Doctoral Fellow, the NIBIB Laboratory of Molecular Imaging and Nanomedicine (LOMIN)
How is it formulated?
A number of nanovaccines were formulated, where each nanovaccine included a distinctive antigen, or the component that activates the immune cells to target a particular type of tumor. The researchers also added Evans blue (EB), a small dye molecule, to each antigen, where the dye molecule gets attached to the albumin inside the body and has been applied for around 100 years in the clinic to investigate albumin binding proteins. The vaccine-EB complex was termed AlbiVax, or albumin-binding vaccine, since it instantly gets attached to albumin. The nanovaccine also comprised of a tiny DNA segment attached to the EB; the DNA functions as a “danger signal” to the immune system and so assists in rendering the immune response highly powerful.
Hence, the entire formulation comprises of a DNA segment and an antigen, both attached to EB. When they are injected, both components get attached to albumin – the reason why the vaccine is regarded to be self-assembling. Then, the albumin carrying the DNA and the antigen distributes both to the lymph nodes, where the antigen stimulates immune cells that particularly attack the tumor, and the DNA improves this stimulation, enhancing the immune attack.
Anti-tumor effects of the AlbiVax nanovaccine
The vaccines were tested on different types of tumors and in different ways. In one such test, tumor-free mice were vaccinated to destroy mouse thymus tumor cells. The mice were vaccinated three times, at 2 week intervals.
On the 70th day, a sizeable dose of the tumor cells was administered into seven vaccinated mice as a trial. Out of the seven mice, five survived for over 4 months. The five mice that survived were administered again with another sizeable dose of tumor cells. Out of the five, four mice survived for over 6 months. Blood tests demonstrated that 4 months following the final immunization, the immune cells particularly targeting the thymus tumor cells were still circulating in the mice.
The researchers also engineered an AlbiVax nanocomplex vaccine against a human colon cancer cell line. The human colon cancer cells are administered into the mice where they formed tumors in different organs. Majority of the tumors were formed in the lung, making the system a better model for vigorous colon cancer cells metastasized to the lung. The vaccine was administered to the mice 6 days following the formation of lung tumors. As a part of the experiment, an antibody known as anti-PD-1 was used to treat the mice, to nullify the impacts of the protein PD-1 that is seen on the surface of specific tumors and functions to slow down the immune attack. Here, the combination of anti-PD-1 and the nanovaccine led to absolute regression of lung tumors in six out of the 10 mice over a period of 4 months.
The researchers are specifically confident about the enduring immunity that they could induce by using the AlbiVax system, as corroborated by persistent, powerful anti-tumor activity for nearly 6 months. This was the lengthiest time point investigated in these experiments and demonstrates an important part of the lifetime of a mouse—which is nearly 2 years.
Albumin is an interesting protein and it has been studied for over 40 years for drug delivery using different technologies. Compared with other albumin-binding technologies, our proprietary technology has been developed using clinically safe EB, making it very promising for eventual clinical translation. By simply synthesizing albumin-binding vaccines, our technology can be applied to virtually any molecular vaccine or molecular therapeutics.
Guizhi Zhu
Xiaoyuan Chen, PhD, the senior author of the study, is the Chief of the NIBIB Laboratory of Molecular Imaging and Nanomedicine. Additional laboratories at NIBIB in Bethesda, MD, that contributed to the study include the Advanced Imaging and Microscopy Resource, the Laboratory of Cellular Imaging and Macromolecular Biophysics, and the Section on High Resolution Optical Imaging. The Laboratory of Molecular Immunology at the National Institute of Allergy and Infectious Diseases; the Cancer and Inflammation Program; National Cancer Institute, Frederick, MD; and the Advanced Imaging and Microscopy resource, NIH, Bethesda, are the additional NIH partners.
The Molecular Imaging Center, Department of Radiology, Keck School of Medicine, University of Southern California, Los Angeles; the School of Engineering, China Pharmaceutical University, Nanjing, China; the State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, Center for Molecular Imaging and Translational Medicine; and the School of Public Health, Xiamen University, Xiamen, China, are the additional partnering laboratories.