Aug 8 2019
The age-old fact that gold can be utilized to do things was something that philosophers had never dreamed of.
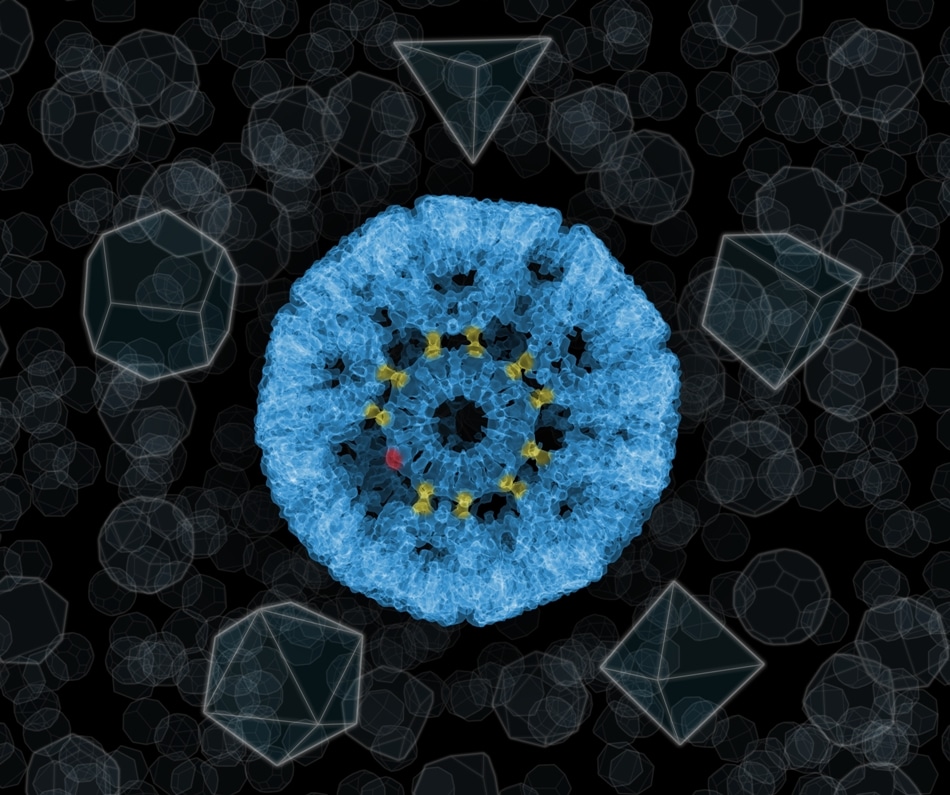 The “impossible” sphere, i.e. a molecular nanocage of 24 protein rings, each of which has an 11-sided structure. The rings are connected by bonds with the participation of gold atoms, here marked in yellow. Depending on their position in the structure, not all gold atoms have to be used to attach adjacent proteins (an unused gold atom is marked in red). (Image credit: UJ, IFJ PAN)
The “impossible” sphere, i.e. a molecular nanocage of 24 protein rings, each of which has an 11-sided structure. The rings are connected by bonds with the participation of gold atoms, here marked in yellow. Depending on their position in the structure, not all gold atoms have to be used to attach adjacent proteins (an unused gold atom is marked in red). (Image credit: UJ, IFJ PAN)
Now, the existence of “gold glue”—bonds involving gold atoms that can permanently bond protein rings—has been confirmed by the Institute of Nuclear Physics of the Polish Academy of Sciences in Cracow.
The bonds, which are deftly used by an international research team, have made it viable to build molecular nanocages whose structure is incomparable in nature or even in mathematics, even today.
For years, the realm of science has shown a considerable interest in molecular cages which indeed has a valid reason. Chemical molecules, such as those that would enter into chemical reactions under typical conditions, can be held within their hollow interiors.
The enclosed compound’s particles, separated by cage walls from the environment, do not have anything to bond with. These cages can hence be used for transporting drugs into a cancer cell in a safe manner and releasing the drug only when they are within it, for example.
Molecular cages are polyhedra composed of smaller “bricks,” which are often protein molecules. The bricks cannot exist in any shape. For instance, if individuals want to construct a molecular polyhedron using just objects with the outline of an equilateral triangle, then geometry would restrict them to just three solid figures—an icosahedron, an octahedron, or a tetrahedron. To date, no other structural possibilities exist.
Fortunately, Platonic idealism is not a dogma of the physical world. If you accept certain inaccuracies in the solid figure being constructed, you can create structures with shapes that are not found in nature, what’s more, with very interesting properties.
Dr Tomasz Wrobel, Institute of Nuclear Physics, Polish Academy of Sciences
Dr Wrobel is part of the international research team who has recently done the “impossible”—the team constructed a sphere-shaped cage out of 11-walled proteins.
Researchers from the team of Professor Jonathan Heddle from the Malopolska Biotechnology Centre of the Jagiellonian University in Cracow and from the Japanese RIKEN Institute in Wako are the main authors of this breakthrough success. The results of the study have been reported in Nature magazine.
Scientists from universities in Waterloo (Canada), Durham (Great Britain), Osaka and Tsukuba (Japan), and other research centers took part in the study.
Each wall of the novel nanocages was created by a protein ring from which 11 molecules of cysteine stuck out at fixed intervals. The “glue”—that is, the gold atom”—was planned to be fixed to the sulfur atom present in each cysteine molecule.
In the ideal conditions, the glue may bind with one more sulfur atom, in the cysteine molecule of the subsequent ring. This way, a permanent chemical bond will be created between the two rings. However, it is not known whether the gold atom under these conditions would really create a bond between the rings.
In the Spectroscopic Imaging Laboratory of IFJ PAS we used Raman spectroscopy and X-ray photoelectron spectroscopy to show that in the samples provided to us with the test nanocages, the gold really did form bonds with sulphur atoms in cysteines. In other words, in a difficult, direct measurement, we proved that gold ‘glue’ for bonding protein rings in cages really does exist.
Dr Tomasz Wrobel, Institute of Nuclear Physics, Polish Academy of Sciences
Treating each gold atom as a stand-alone clip can make it possible to fix another ring. It is not always necessary to use all the clips and this realization initiates the road to “impossible.” Hence, even when all the rings of the novel nanocages remain physically the same, they bind with their neighbors with a varying number of gold atoms based on their position in the structure, and thus work as polygons with different numbers of vertices. The researchers presented 24 nanocage walls that were held together by 120 gold atoms. The inner and outer diameters of the nanocage were 16 and 22 nm, respectively.
Gold atoms should be used as a binder for nanocages because of their potential applications. In previous molecular structures, proteins were fixed together using several weak chemical bonds. The intricacy of the bonds and their resemblance to the bonds that account for the existence of the protein rings did not enable accurate control over the decomposition of the nanocages. However, this does not happen in the new structures.
While gold-bonded nanocages are thermally and chemically stable (for instance, they endure hours of boiling in water), gold bonds are known to be sensitive to increased acidity. This increase causes the nanocage to decompose in a controlled way and the contents within them can be discharged into the environment. Since the interior of the cells has greater acidity than the exterior, gold-bonded nanocages offer a suitable solution for biomedical applications.
The “impossible” nanocage demonstrates a qualitatively new method for building molecular cages, with gold atoms functioning in the role of loose clips. In the future, the demonstrated flexibility of the gold bonds will help develop nanocages with features and sizes accurately customized to particular requirements.