Jan 20 2020
Researchers have not been able to perform any experiment that can test whether the behavior of molecules actually obeys the same trend as seen at human scale. Led by Simone Napolitano, a research team from the Faculty of Sciences, Université libre de Bruxelles (ULB) has demonstrated that large molecules move faster when they are close to rougher surfaces at the nanometric scale.
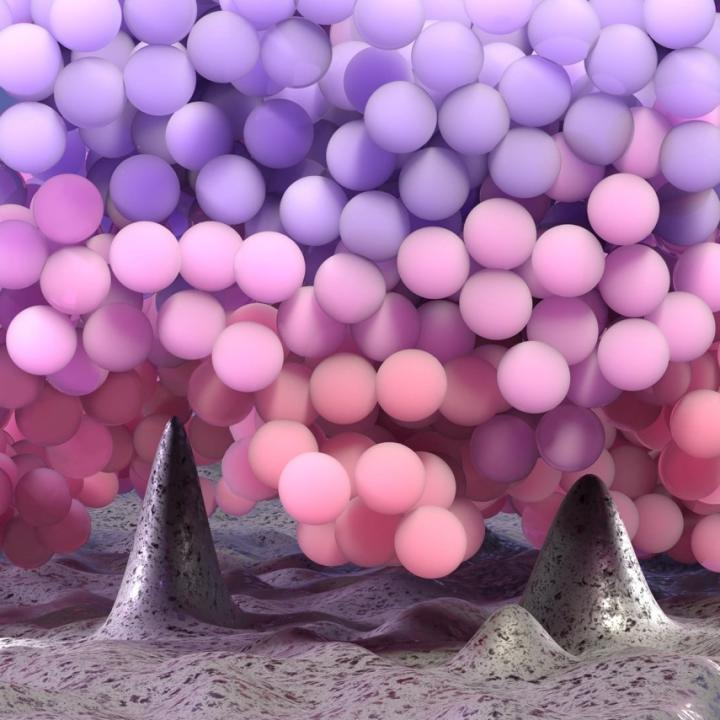 3D rendering of polymer chains near the asperities of a rough substrate. Faster molecules were pictured by warmer colors. Image Credit: ©ULB.
3D rendering of polymer chains near the asperities of a rough substrate. Faster molecules were pictured by warmer colors. Image Credit: ©ULB.
The study outcomes have now been reported in Physical Review Letters. Their experiments evidently show that the common notion that irregularities on surfaces permit molecules to better stick on a surface is actually incorrect.
Upon reducing the surface roughness size, that is, the average distance between the tiny valleys and hills on a material’s surface, to a few nanometers (where 1 nm = one billionth of 1 m), molecules of P4ClS, a kind of polymer, start to move faster.
It is not easy to observe molecular motion: molecules tend to move very fast (even up to 1 million or more steps per second) and their displacements are extremely small to be detected using microscopes. It is even more challenging to carry out such experiments on a rough surface due to its uneven character and since it is difficult to adjust the size and distribution of the irregularities on the surface.
The ULB researchers have been able to create a rough surface of aluminum, through the evaporation of the metal in a controlled manner. They measured the speed at which the molecules move by applying weak electric fields and recording how quickly the molecules react to the stimulus.
Fascinatingly, the researchers observed that molecules existing close to a rough substrate act as if fewer neighbors surrounded them. This elucidates why they accelerate rather than slowing down. This trend is in stark contrast to the estimations of computer simulations, which suggested that molecules move slower close to a rough wall.
In contrast to what was considered in simulations, polymer molecules do not like to sit close to the rough substrate. Due to the way these molecules intend to arrange themselves in the space, they choose to move away from the asperities. The fewer molecules that exist close to the asperities do not contact much with the wall, can have more free volume and, as a result, they move faster.
The ULB researchers have shared the study outcomes with a team of theoreticians from Dartmouth College (USA), under Jane Lipson, thereby finding a firm connection between the way valleys and hills are organized on a rough surface and the way molecules move.
The theoreticians have demonstrated that even a slight change in the free volume surrounding a molecule triggers an immense boost in mobility, and the prediction of their calculations are in good accordance with the experiments.
This study reveals that researchers’ existing knowledge about interfaces is not valid. Therefore, this newly detected molecular trend has a great effect at the level of fundamental science. The research by the ULB researchers could be leveraged on several applications.
For nearly a decade, a number of research teams have demonstrated that properties of various thin coatings such as flow, the potential to repel or retain water, the velocity of crystal formation—rely on the number of contacts between the film and its supporting substrate. To date, this number could be altered only by changing the type of molecules at the interface, which usually involves complex chemical reactions.
The study outcomes demonstrate that the performance of nanomaterials can be simply tailored by modifying the roughness of the surface. Thus, this technique enables the polymer layer to be controlled without touching it, similar to being done with remote control!
The FNRS, the Federation Wallonie Bruxelles, and the National Science Foundation supported this study.