Sep 8 2020
A new technique for recycling silicon has been developed by researchers from Skoltech and their collaborators from Lomonosov Moscow State University (MSU). The study was reported in ACS Sustainable Chemistry & Engineering.
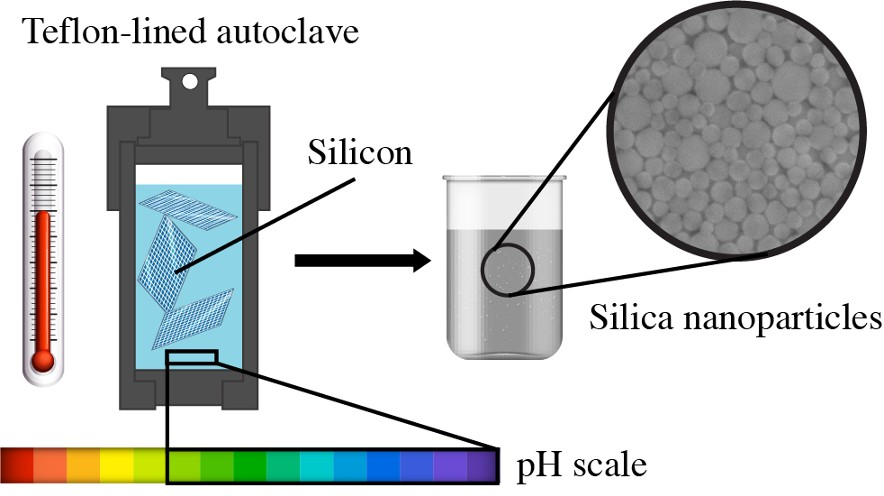 Image Credit: Skolkovo Institute of Science and Technology.
Image Credit: Skolkovo Institute of Science and Technology.
Most of the solar panels developed in ever-increasing numbers are based on silicon. Solar panels with a lifetime of 25 to 30 years tend to deteriorate and generate less electricity as time passes, which makes recycling of the silicon waste a hot topic.
If nothing is done recycle silicon waste, by 2050, the Earth will be dumped with 60 million tons of used photovoltaic panels. The conversion of silicon into nanoparticles of silicon oxide has crucial implications for the environment by addressing the silicon waste recycling issue and offering a new nanoparticle source for a range of applications in science and industry.
Led by Stanislav Evlashin, a senior research scientist from the Skoltech Center for Design, Manufacturing and Materials (CDMM), a team of researchers exhibited a simple, 100% efficient technique to convert silicon wafers into nanoparticles in an aqueous solution. This finding could help create an eco-friendly method of recycling silicon without the use of toxic chemicals.
The new conversion process is controllable and allows the sizes of the nanoparticles to be controlled, which can be subsequently reused in medicine, optics, photonics, and other fields.
The used panels are converted into nanoparticles using hydrothermal synthesis in an aqueous environment. The good thing about this process is that nanoparticle sizes can be controlled within a range of 8 to 50 nm without using a lot of equipment.
Stanislav Evlashin, Senior Research Scientist, Center for Design, Manufacturing and Materials, Skolkovo Institute of Science and Technology
Three types of silicon wafers were used by the team in the experiment: N-type (Nitrogen-doped), P-type (Phosphorus-doped), and HR (High resistivity). Their theoretical estimations were based on the density functional theory and demonstrated the formation of Si-H bonds form on the surface of HR-plates, even when ammonia was not used as a catalyst.
Moreover, the reaction can be accelerated with the help of additives such as boron and phosphorus dopants, as well as molecular defects (in the case of solar panels).
The vast majority of methods used to synthesize silicon oxide nanoparticles are based on the bottom-up approach and, therefore, use alkoxides as a precursor. By contrast, ours is a top-down method that uses bulk silicon as a source, which creates a wealth of advantages, such as simplicity, scalability, and controllable particle size distribution.
Julia Bondareva, PhD Student, Skolkovo Institute of Science and Technology
Bondareva added that “Temperature and hydrolysis time are the key parameters of the synthesis that influence the particle size distribution. We noticed that an increase in pH has a strong effect on the particle formation rate. This is why we used ammonia which made the reaction in an aqueous medium much faster.”
We decided to figure out how nanoparticles form in the process, among other things. To do this, we used a heterogeneous nucleation model with a finite number of nucleation centers distributed over the silicon source surface.
Timur Aslyamov, Senior Research Scientist, Skolkovo Institute of Science and Technology
Besides pure silicon, the researchers also used Si-ITO heterostructure-based industrial solar panels, which behaved similarly to silicon panels and were successfully transformed into nanoparticles. This study is considered to be a crucial landmark in achieving eco-friendly, safe recycling of silicon waste and generating new sources of silicon oxide nanoparticles.
Journal Reference:
Bondareva, J. V., et al. (2020) Environmentally friendly method of silicon recycling: synthesis of silica nanoparticles in an aqueous solution. ACS Sustainable Chemistry & Engineering. doi.org/10.1021/acssuschemeng.0c03783.