Mar 3 2021
NUS scientists have devised a new method for the synthesis of nanographene molecules with a high product yield for the development of next generation quantum devices.
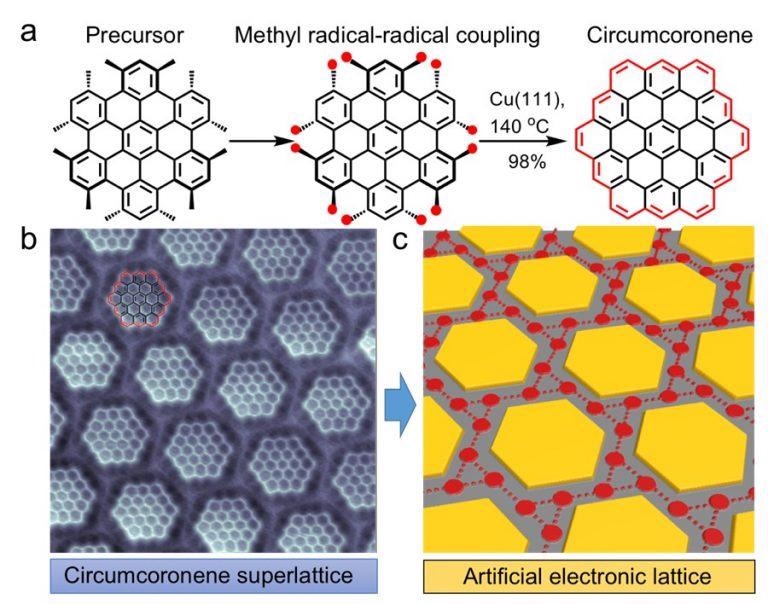 Figure shows the transformation from the precursor molecule to the atomically-precise circumcoronene superlattice. (a) The precursor molecule undergoes a cyclodehydrogenation chemical process with the use of copper (111) substrate which forms the circumcoronene. (b) High-resolution image of the circumcoronene superlattice obtained by using a non-contact atomic force microscopy with a cobalt tip. (c) Schematic illustration of the chiral Kagome-honeycomb lattice. [Image Credit: Science Advances]
Figure shows the transformation from the precursor molecule to the atomically-precise circumcoronene superlattice. (a) The precursor molecule undergoes a cyclodehydrogenation chemical process with the use of copper (111) substrate which forms the circumcoronene. (b) High-resolution image of the circumcoronene superlattice obtained by using a non-contact atomic force microscopy with a cobalt tip. (c) Schematic illustration of the chiral Kagome-honeycomb lattice. [Image Credit: Science Advances]
On-surface chemical reactions have shown promising potential in the synthesis of new organic functional materials such as atomically-precise nanographenes. The core concept of this strategy relies on the rational design of specific molecular precursors, which subsequently undergo chemical transformation along certain reaction pathway towards the desired product. The electronic, magnetic and optical properties of these nanographene molecules can be precisely tuned for the development of next-generation quantum devices. Unfortunately, conventional surface-assisted synthetic routes often involve a series of cascade reactions with competing reaction pathways. This inevitably leads to the formation of numerous undesired products and lowers the yield. The limited yield of the targeted products poses a challenge for practical applications of the nanographenes.
A NUS research team led by Prof Jiong LU, in collaboration with Prof Jishan WU’s research group, both from the Department of Chemistry, NUS has developed a route for synthesizing the hexagonal zigzag-edged nanographene, known as circumcoronene, on a copper (111) substrate. The reaction route relies on the robust dehydrogenative coupling of the methyl groups at the adjacent sites of the rationally-designed precursor molecules, followed by the ring closure reactions on the metallic substrate. This forms the elusive circumcoronene molecule consisting of 19 fused benzene rings (Figure (a)). Importantly, such a synthetic route allows for an ultra-high yield of the reaction product (up to 98%), which has not been attained to date.
The electrostatic interactions between the large number of circumcoronene molecules and the copper substrate enabled the molecules to self-assemble into extended superlattices. This was observed by the team using bond-resolved scanning probe microscopy measurements (Figure (b)). The researchers demonstrate that the unique hexagonal zigzag topology of circumcoronenes, along with their periodic electrostatic landscape, confines the two-dimensional (2D) electron gas on the copper (111) surface. This creates a chiral electronic Kagome-honeycomb lattice with two emergent electronic flat bands (Figure (c)). This arrangement of the circumcoronene molecules in a regular grid of hexagons and triangles can be particularly interesting in a wide range of condensed matter physics because of their favourable potential in the realisation of a variety of exotic many-body phenomenon, including anomalous quantum Hall states, Wigner crystallisation, and topological insulating transitions.
Prof Lu said, “Our findings open up a new route for the ultra-high yield synthesis of nanographene and atomically-precise fabrication of synthetic two-dimensional lattices with unique electronic properties for future technological applications.”