Apr 15 2021
A new self-assembling nanomaterial designed by biomedical engineers from Duke University stimulates major cells in the immune system that can help reduce damage caused by inflammatory disorders.
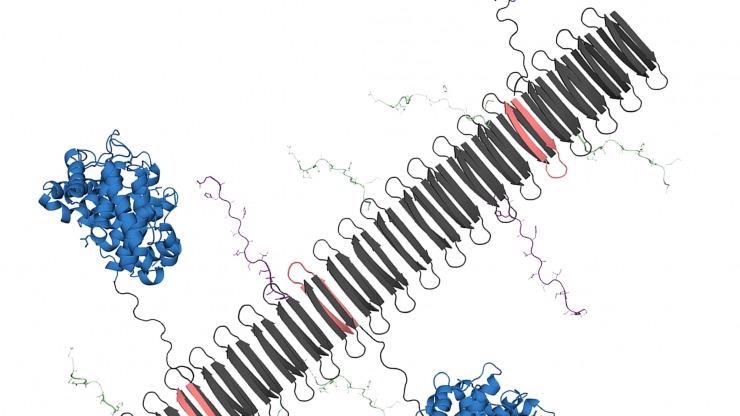 The graphic shows the peptide nanofiber bearing complement protein C3dg (blue) and key components of the TNF protein, which include B-cell epitopes (green) and T-cell epitopes (purple). Image Credit: Chelsea Fries.
The graphic shows the peptide nanofiber bearing complement protein C3dg (blue) and key components of the TNF protein, which include B-cell epitopes (green) and T-cell epitopes (purple). Image Credit: Chelsea Fries.
In psoriasis mouse models, the nanofiber-based medication has been demonstrated to reduce the damaging inflammation as successfully as a gold-standard treatment.
The overproduction of signaling proteins, known as cytokines, is one of the hallmarks of inflammatory disorders, such as psoriasis, Crohn’s disease and rheumatoid arthritis. These signaling proteins are known to cause inflammation.
TNF is a type of protein that is one of the most important inflammatory cytokines. At present, the optimal treatment for these disorders involves the use of monoclonal antibodies, which are manufactured antibodies and have been developed to target and kill TNF and thus decrease inflammation.
While monoclonal antibodies have facilitated a better treatment of inflammatory disorders, this treatment has certain disadvantages, such as high costs and the necessity for patients to routinely inject themselves.
Most importantly, the medications also have unstable efficacy because they may occasionally not work at all, or ultimately stop working as the body learns to produce antibodies that can kill the manufactured medication.
To overcome these problems, scientists have been studying how the immune system could be taught by immunotherapies to create its own therapeutic antibodies that can particularly reduce inflammation.
We’re essentially looking for ways to use nanomaterials to induce the body’s immune system to become an anti-inflammatory antibody factory. If these therapies are successful, patients need fewer doses of the therapy, which would ideally improve patient compliance and tolerance. It would be a whole new way of treating inflammatory disease.
Joel Collier, Professor of Biomedical Engineering, Duke University
In their latest article, which was published online in the Proceedings of the National Academy of Sciences on April 5th, 2021, Collier and Kelly Hainline, a graduate student in the Collier lab, explained how new nanomaterials could organize into long nanofibers that feature a specialized protein, known as C3dg. Such fibers can then trigger the B-cells in the immune system to generate antibodies.
“C3dg is a protein that you’d normally find in your body. The protein helps the innate immune system and the adaptive immune system communicate, so it can activate specific white blood cells and antibodies to clear out damaged cells and destroy antigens,” stated Hainline.
Since the C3dg protein is capable of interfacing between different types of cells in the immune system and triggering the production of antibodies without causing inflammation, scientists have been investigating how this protein could be utilized as a vaccine adjuvant, which is another protein that can help increase the immune response to a required pathogen or target.
In their novel nanomaterial, both Hainline and Collier tested this concept by weaving crucial fragments of the C3dg protein with TNF components into nanofibers. While the C3dg protein would activate the B-cells to produce antibodies, the TNF components would give a blueprint of what the antibodies had to look for and destroy.
When Kelly assembled the C3dg protein and key portions of TNF into these nanofibers, she saw that there was a strong B-cell response, which means there was an increased production of antibodies that targeted TNF. In standard mouse models of inflammation, mice experience a temperature change where their internal temperature will drop. But when Kelly delivered her C3dg nanofibers, it was highly protective, and the mice didn’t experience an inflammatory response.
Joel Collier, Professor of Biomedical Engineering, Duke University
When the new nanomaterial was tested in the psoriasis mouse model, the researchers observed that the nanofibers carrying the C3dg protein were equally effective as a monoclonal antibody treatment. And since the C3dg protein is generally found in the body, it was removed from the system by anti-drug antibodies.
After examining the psoriasis model, the team made an unexpected finding—the C3dg protein was not simply triggering the production of antibodies in the B-cells but was also affecting the T-cell response.
We observed that nanofibers that only contained the C3dg components without the TNF components still showed a therapeutic benefit to our models, which was surprising. But I think the most significant discovery was seeing a beneficial T-cell response that was activated by a protein you’d naturally find in your body. That kind of response had been seen before with other proteins, but we haven’t seen any reports of people using that response with C3dg.
Kelly Hainline, Graduate Student, Duke University
For their subsequent steps, the researchers hope to further study the mechanisms behind this activation of beneficial T-cells. The team will also perform more experiments to investigate the response to analogous nanomaterials in models of rheumatoid arthritis.
“We’re still learning about this T-cell response, and we’re trying to understand how it’s involved. Ultimately, we’d love to see if C3dg can be used as a universal component in multiple different therapies against inflammation, especially if we can swap out the TNF segments with a different target. This work clearly indicates that nanomaterials involving C3dg warrant further development as immunotherapies,” concluded Collier.
The study was financially supported by the National Institutes of Health (NIBIB 5R01EB009701) and Duke University, an NIH Training Grant (T32GM008555), the NSF Graduate Research Fellowship Program (DGE-1644868), and the North Carolina Biotechnology Center (2017-IDG-1018).
Journal Reference:
Hainline, K. M., et al. (2020) Modular Complement Assemblies for Mitigating Inflammatory Conditions. Proceedings of the National Academy of Sciences. doi.org/10.1073/pnas.2018627118.