Reviewed by Alex SmithSep 8 2021
Researchers at the University of Central Florida have developed a new nanomaterial capable of repelling water and staying dry even when submerged underwater.
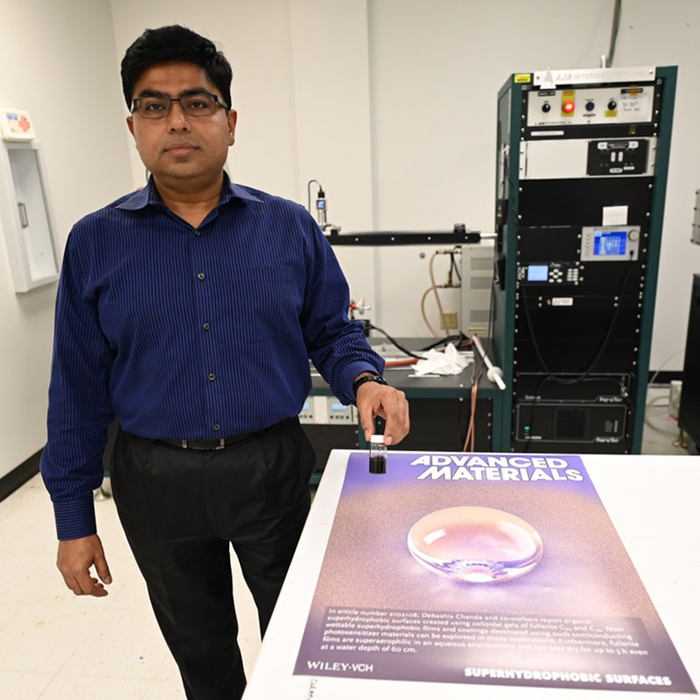 Debashis Chanda, a professor at UCF’s NanoScience Technology Center, led the team that created these novel superhydrophobic films and coating from nanomaterials. He was inspired by the nature and evolution of certain plants and biological species. Image Credit: University of Central Florida.
Debashis Chanda, a professor at UCF’s NanoScience Technology Center, led the team that created these novel superhydrophobic films and coating from nanomaterials. He was inspired by the nature and evolution of certain plants and biological species. Image Credit: University of Central Florida.
The discovery could lead to the development of more efficient water-repellent surfaces, fuel cells and electronic sensors to detect toxins. The study was featured in the journal Advanced Materials.
The research team was led by Debashis Chanda, a professor at UCF’s NanoScience Technology Center. The team had developed novel superhydrophobic films and coatings using nanomaterials. Chanda was inspired by the nature and evolution of particular plants and biological species.
Being water repellent or hydrophobicity is nature’s tool to protect and self-clean plants and animals against pathogens like fungi, algae growth and dirt accumulation. We took our cues from the structure of a lotus leaf and synthesized nanostructured materials based on molecular crystals of fullerenes.
Debashis Chanda, Professor, NanoScience Technology Center, University of Central Florida
Fullerenes (C60 and C70) are developed by bundling carbon molecules, which are the fundamental building block of the universe. Carbon evolves in various forms. Under exceptional conditions, 60 or 70 such carbon molecules can combine to form a cage-like closed structure, known as fullerenes. These cages are capable of stacking on each other to create tall crystals named fullerites.
According to Chanda, a super water-repellent state is triggered by placing a drop of gel produced from a fullerite on any surface. The distinct cage-like structure of the gel does not impact the original material under treatment, which implies that they preserve their exclusive functional characteristics.
This implies that the new super surface can be used for electrocatalysis, splitting water, hydrogen generation or bacterial disinfection, which can all be produced in fluid environments.
For example, the new gel makes splitting electrocatalysis easier, which could lead to more efficient fuel cells. The same gel can lead to better electron acceptors, which are key in developing highly sensitive detectors and sensors for toxic gases. There is a lot of potential. It is quite exciting.
Debashis Chanda, Professor, NanoScience Technology Center, University of Central Florida
Most of the previously observed hydrophobic surfaces were successfully formed by designing microscopic patterns that involve complicated lithography or etching processes that cannot be conducted on all surfaces. Moreover, all of the previously developed hydrophobic surfaces cannot remain submerged underwater for more than a few minutes at a specific water depth.
We found that fullerite films display extreme water repellency regardless of direction of water flow and even under continuous flow of water over them. Even when they are submerged at 2 feet of water for several hours, the films remain dry. We even found that they can capture and store gases underwater in the form of plastrons — a form of trapped bubbles mimicking the miraculous alkali fly of California’s Mono Lake.
Debashis Chanda, Professor, NanoScience Technology Center, University of Central Florida
Rinku Saran, a post-doctoral fellow in Chanda’s lab and lead author of the study, expresses his excitation regarding the potential of the project.
Saran Stated, “Because these superhydrophobic surfaces are created in a very facile and easy process using pure carbon fullerenes we anticipate they can be exploited in many experiments and real-life applications.”
The other co-authors are David Fox, a doctoral student at NanoScience Technology Center, and Professor of Chemistry Lei Zhai.
Chanda has a joint appointment in UCF’s Department of NanoScience Technology Center, the Department of Physics, and the College of Optics and Photonics. Chanda received his doctorate in photonics from the University of Toronto and worked as a postdoctoral fellow at the University of Illinois at Urbana-Champaign before joining UCF in 2012.
This work at the University of Central Florida was supported by the U.S. National Science Foundation grant and the UCF Preeminent Postdoctoral Program (P3).
Journal Reference:
Saran, R., et al. (2021) Organic Non-Wettable Superhydrophobic Fullerite Films. Advanced Materials. doi.org/10.1002/adma.202102108.