The existence of microbial antigens and other impurities mistakenly introduced during the development and purification of bionanopharmaceutical devices can stimulate the innate immune system, as described in a paper published in the journal Molecules.
Study: Innate Immunity Modulating Impurities and the Immunotoxicity of Nanobiotechnology-Based Drug Products. Image Credit: peterschreiber.media/Shutterstock.com
An immediate but largely non-specific local immune reaction including both biochemical and molecular components initiates the body's first "innate" defense against foreign armies.
Trained immunity is a non-specific, T-cell self-sufficient innate immunity that relies primarily on macrophage activation and pro-inflammatory cytokine secretion for long-term functional reconfiguration of the innate immune cell response instead of the epigenetic hybridization required by innate and adaptive immunity.
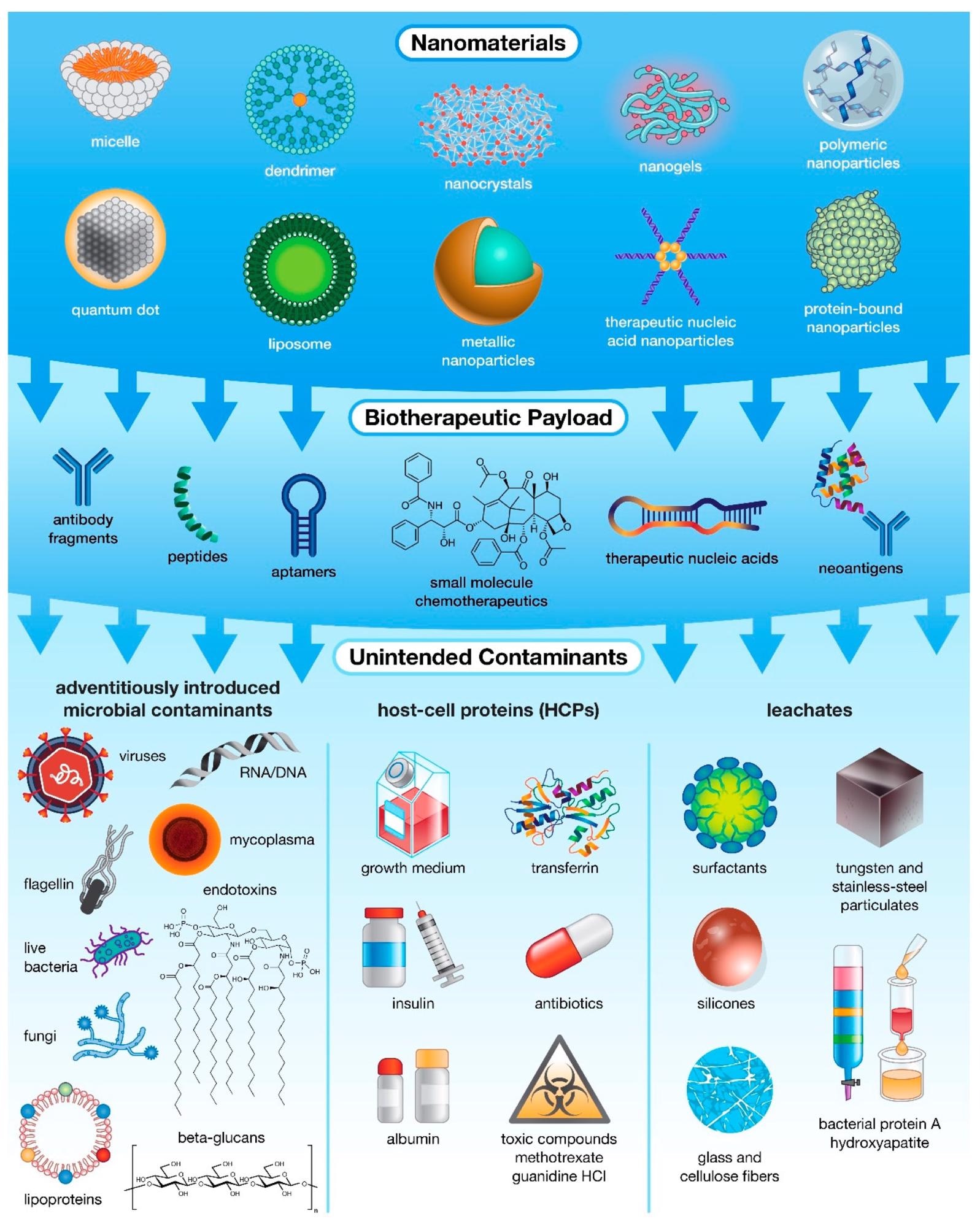
The many levels of possible unintended contamination in drug products. While most often associated with microbial contamination, unintended impurities can actually be introduced into pharmaceutical products from multiple sources, including raw materials and specialized host-cell reagents, and at various stages of production, ranging from fabrication and payload encapsulation in nanocarriers to purification of the final formulation. Innate Immunity Modulating Impurities or IIMIs. Image Credit: Holley, C., and Dobrovolskaia, M.
Due to the high financial and social expenses of medicine development, research, and approval, it is critical that any prospective product "failure" is not caused by the accidental inclusion of innate immunity modulating impurities IIMIs
Activated phagocytes produce simultaneously stimulatory as well as inhibitory cytokines in the influence of IIMIs to stimulate and control the immune response.
Chemokines are the most diversified family of cytokines, with roles ranging from cell migratory regulation (e.g., recruiting and activation of local neutrophils and basophils to the infection site) through embryogenesis, innate and adaptive body's immune function and structure, and cancer metastasis.
Cytokine to Boost Vaccine Effectiveness
In most cases, cytokine-driven immunostimulation is beneficial, such as when it is activated by adjuvants to boost vaccine effectiveness.
Immunological stimulation that is unanticipated or uncontrolled, particularly in the presence of therapeutic substances, causes unwanted cellular immune responses and antibody formation in reaction to the medicinal product.
Immunotoxicity is defined as "any unfavorable effect on the structure or function of the immune mechanism, or other systems influenced by the same biologic mediators as a result of immune response malfunction."
It is further divided into three categories based on the intensity of the response: non-specific immunostimulation, uncontrolled hypersensitivity that causes tissue injury, and immunosuppression.
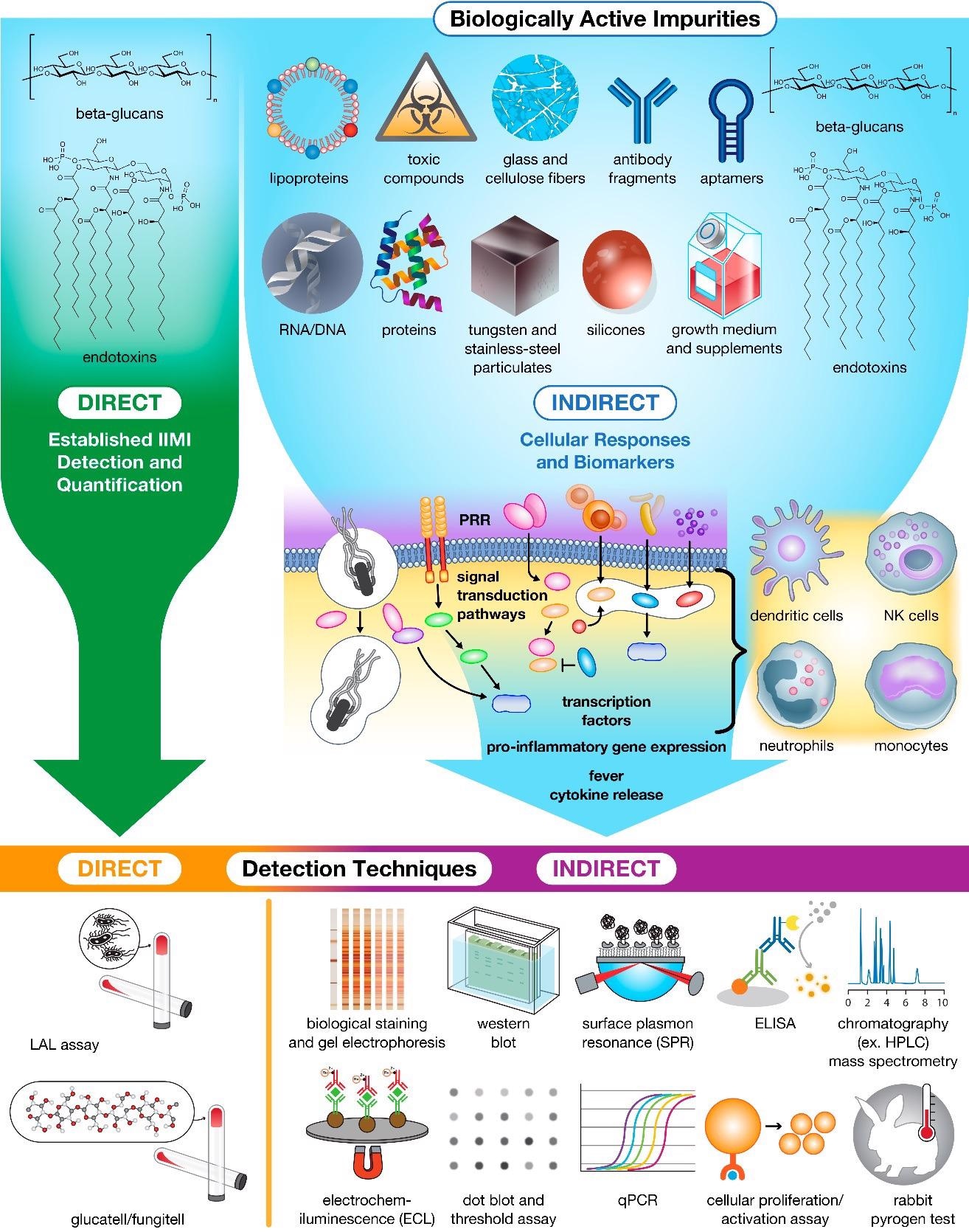
Impurities in drug products trigger innate cellular responses and produce biomarkers for bioassay detection and Quantification. Currently, only β-glucans and endotoxins can be detected and quantified directly using specialized assays. The remaining population of impurities must instead be detected and quantified indirectly using downstream biomarkers (e.g., proteins, peptides, and nucleic acids) and immune cell activation as hallmarks of contamination. Image Credit: Holley, C., and Dobrovolskaia, M.
Application of Nanotechnology in Biomedical Research
When compared to classically formulated variants of such prescription medications, the use of nanotechnology is becoming a popular method for reducing drug immunotoxicity whilst also improving medicinal solubility, biodistribution, and cell-specific distribution. However, several nanocarriers have been shown to have immunomodulatory properties.
For example, RNA nanoparticles have been found to increase inflammation by inducing pro-inflammatory cytokine release. The raw materials used to make nanoplatforms can have a variety of immunological impacts, either as a result of contamination or because of the chemical features of the material.
Certain nanomaterials, including lipid-based nanocarriers and carbon nanotubes, are immunostimulatory, causing cytokine production and inflammation.
NanoFormulations Testing on IIMIs
The rabbit pyrogen test (RPT) became the bioassay used to identify microbial contamination. It detects pyrogens, as well as any contaminants that cause a histamine reaction, chills, fever, and other inflammation side effects.
As the rabbit pyrogen test identifies all pyrogens, it has a high level of unpredictability, is costly, and requires significant animal usage for tests.
As issues with beta-glucan and endotoxin identification in nanoformulations arise from excipient-, carrier-, or drug-mediated external interference, sources of interferences and techniques to overcome them have been discovered. Here, direct detection methods are often utilized.
For an efficient test, a suitable biomarker can be any chemical with a beneficial attribute, such as a mechanical by-product, that can be measured or assessed, either direct or indirect, and utilized as an indication of a normal biological, pathological, or pharmacological condition.
Recent experiments have placed focus on the in vitro and in vivo effects of IIMIs because as the long-term objective of these investigations is to prevent human immunotoxicity and probable immunogenicity.
These biological tests detect immune cell growth and proliferation or measure quantities of released innate immunity biomarkers, which may help to prime immune cells and contribute to immunogenicity.
Future Research on IIMIs
The FDA's mandated panel of IIMIs for measurement should be broadened to include a far larger range of impurities, such as microbial antigens that may trigger additional innate immune pathways, popular manufacturing leachates and solvents, and hazardous chemicals needed to keep host cells alive.
The utilization of a single high-throughput platform designed to detect a large panel of indicators from the same class (proteins, small molecules, or nucleic acids) simultaneously, such as multiplex MS, ELISAs, or genomic arrays, should be used to standardize data across trials and laboratories. Broader nanoassortment of cytokines can be applied to make the data more complete.
Continue reading: Why Nanotoxicology Should be the First Step Towards a Nanotechnology Future.
Reference
Holley, C., and Dobrovolskaia, M. (2021). Innate Immunity Modulating Impurities and the Immunotoxicity of Nanobiotechnology-Based Drug Products. Molecules 26(23). Available at: https://www.mdpi.com/1420-3049/26/23/7308
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.