An article published recently in the journal npj 2D Materials and Applications describes developments in single-atom catalysts for crystal growth using graphene.
Study: Single-atom catalytic growth of crystals using graphene as a case study. mage Credit: Rost9/Shutterstock.com
Single-atom catalysts have risen to prominence in recent years, with enormous potential applications in the chemical, renewable energy, and eco-friendly fuel storage industries.
What are Catalysts?
In the last few decades, catalysts have played a significant role in the global industry. Catalysts are required in over 90% of biochemical reactions, and there is a constant need to make them more efficient, affordable, and eco-friendly to ensure modern society's long-term sustainability.
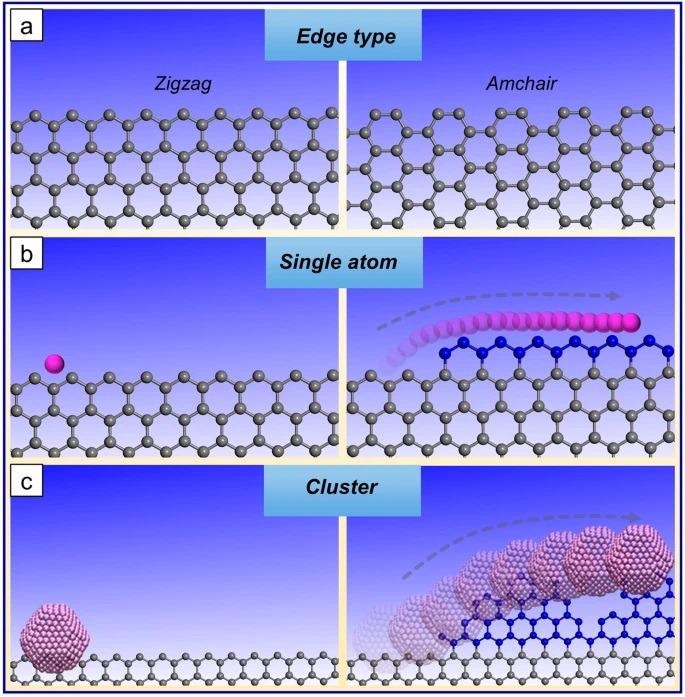
Schematic of edge growth from a single catalyst atom and from a catalyst nanoparticle. Panel a shows the zigzag and armchair edge termination of graphene. Panel b shows a single-atom edge layer being grown at a graphene edge from a single-atom catalyst and panel c shows the typically rough edge formed from a catalyst nanoparticle/cluster at a graphene edge (growth and etching). Image Credit: Yang, X. et al.
In general, there are two types of catalyst reactions: uniform catalysis, in which the catalyst and intermediate products are in the same condition throughout the process, and heterogeneous catalysis, in which the catalyst and intermediate products are in two different phases during the process.
Most commercial catalysts are heterogeneous due to the thermal instability of homogenous catalysts.
The characteristics of heterogeneous catalysts are dependent upon the heterogeneity of the interface and the morphology of the material. The scale of metallic particles is also a crucial component in determining the performance of these catalysts.
However, surface flaws make it very hard to fabricate two similar nanomaterials. One method to address this difficult problem is to decrease the size of a metallic particle to the atomic scale, i.e., a single atom acting as a catalyst, in which scenario interfacial heterogeneity is no longer a concern.
Importance of Single-Atom Catalysts (SACs)
In addition to offering a good specific reaction rate, single-atom catalysts (SAC) lower costs, which is essential for expensive noble metals used in the petroleum industry, medication manufacturing, pollution prevention, and power applications.
A binding agent, such as metal oxide or graphene, is usually used to make single-atom catalysts. SACs offer appealing electrochemical features, such as 100% atomic efficiency (every atom is available for the catalytic process), and the metallic atom-support contact typically increases electron transport, lowering activation energies.
Therefore, the most cost-effective way to obtain excellent reactive groups in catalysts and maintain the required catalytic efficiency is to employ single atoms.
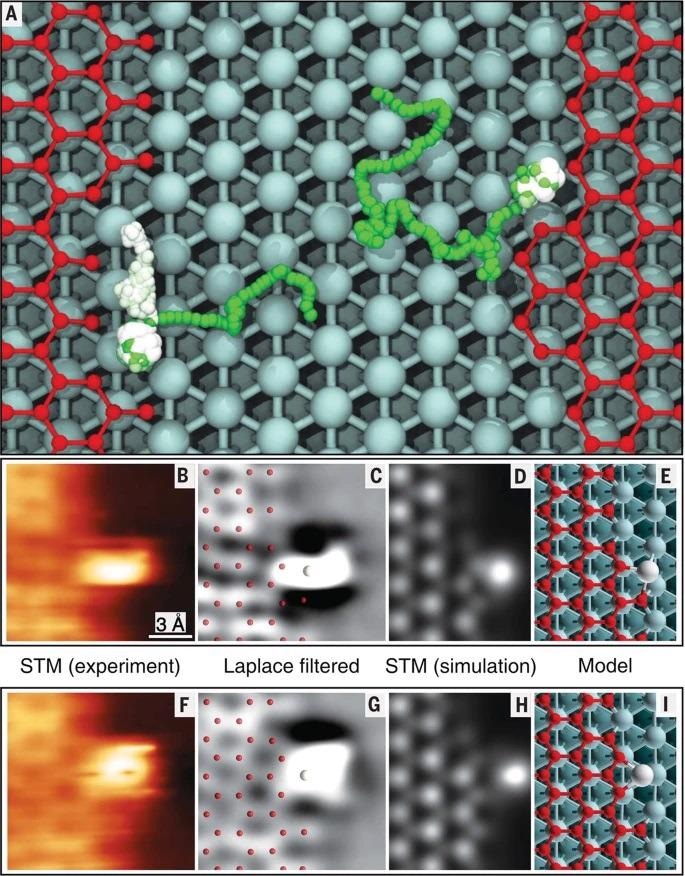
Nickel adatoms at the graphene edges. A Ni adatom diffusing on a surface in a region delimited by graphene z (right) and k (left) edges with kinks. Two representative trajectories obtained by MD simulations with ReaxFF performed at 710 K for 100 ps are shown. Color palette for Ni trajectories: from green (initial position) to white (final position). The final steps are highlighted by increasing the ball size. B–I Short-lived configurations of Ni adatom at k-edge kinks: B and F High-speed STM images from movie S2 (20) (V = 20 mV; I = 7 nA). C and G Laplace-filtered version of images from (B) and (F) with superimposed ball models. D and H constant-height STM simulated images based on the calculated geometries (E and I). Reproduced with permission from the American Association for the Advancement of Science40. Image Credit: Yang, X. et al.
Single Atoms for the Catalytic Growth of Crystalline Materials
At the nanoscale, the utilization of single-atom catalysts for the crystallization process is necessary because the position and orientation of every atom become significant. As a result, to manufacture materials that are beneficial for different industrial applications, it is essential to synthesize them with atomic precision.
Although effective in the microelectronics industry, crystalline materials fabricated using a top-down structuring approach have few other applications because of their atomic imprecision.
In this context, using a single atom catalyst for crystal synthesis provides several benefits as one atom acts as a tool for adding other atoms, and the tool is the same size as the blocks (atoms) used to build the material.
This is particularly true for creating fast-changing 2D materials like graphene and single-walled carbon nanotubes, which have a wide range of applications in bioscience, renewable energy, and electrical devices.
However, the application of single-atom catalysts for atomic-level crystal formation is still in its early stages, and there are several obstacles to overcome. This is due to the fact that identifying atom reaction mechanisms requires very high resolving power.
So far, two imaging devices have shown the ability to offer direct measurements at the atomic level. These are the transmission electron microscope and the scanning electron microscope.
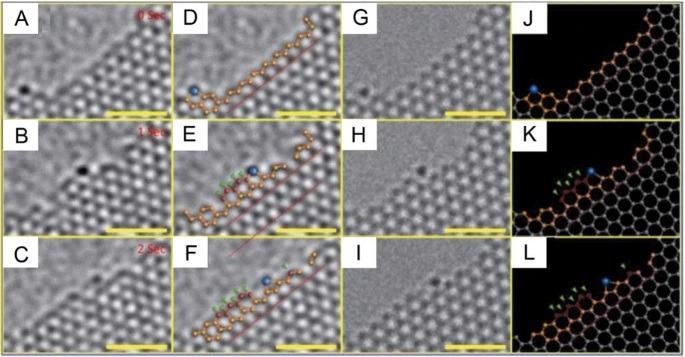
Catalytic growth of graphene by a single Cr atom at the graphene edge under electron beam irradiation. A–C, with partial stick-and-ball models to aid viewing (D)–(F), image simulations of the growth process G–I, and complete stick-and-ball models J–L. The blue ball indicates Cr, whereas red balls and green arrows signify new C atoms. All scale bars are 1 nm. Reproduced with permission from Springer20. Image Credit: Yang, X. et al.
Future Perspective
Based on the results provided by transmission and scanning electron microscopes, it was found that single-atom catalysts can be successfully designed to produce crystalline nanostructures with 2D materials like graphene.
Future research should concentrate on in-depth examinations to better understand the underlying processes and how atomic-level regulation of crystal formation might be accomplished.
This paper introduces a sub-field of single-atom catalysts, namely the SAC growth of crystals using graphene. Undoubtedly, there will be a large number of studies in this interesting new field, and these studies might lead to a considerable expansion in the usage of SACs in many industries.
Continue reading: How are Nanocatalysts Used for Environmental Applications?
Reference
Yang, X. et al. (2021). Single-atom catalytic growth of crystals using graphene as a case study. npj 2D Materials and Applications, 91. Available at: https://www.nature.com/articles/s41699-021-00267-4
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.