Reviewed by Alex SmithApr 27 2022
Novel technologies to convert sunlight into power and fuels will be enabled by new materials. These materials are made possible by the combination of molecules and nanoparticles. These materials’ molecules are excellent at absorbing light and giving electrons to nanoparticles. The nanoparticles then catalyze reactions that make the fuel by moving electrons around. This mechanism, however, does not always operate as researchers want.
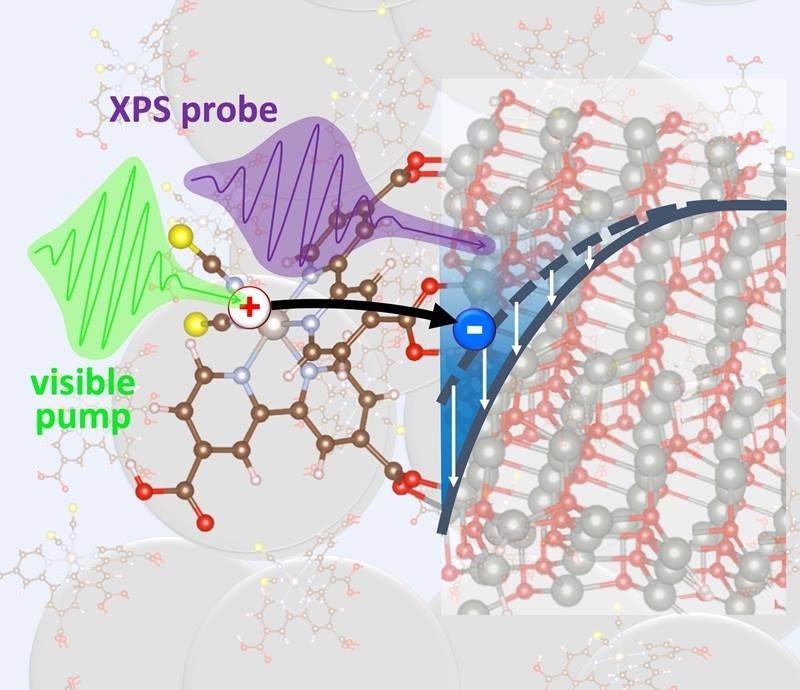 Visible laser pulses excite electrons in molecules attached to a nanoparticle substrate. Short X-ray pulses follow the electrons along their round trip between the molecules and the nanoparticles to show when, where, and why electrons move or get stuck. Image credit: Oliver Gessner and Johannes Mahl, Chemical Sciences Division, Lawrence Berkeley National Laboratory.
Visible laser pulses excite electrons in molecules attached to a nanoparticle substrate. Short X-ray pulses follow the electrons along their round trip between the molecules and the nanoparticles to show when, where, and why electrons move or get stuck. Image credit: Oliver Gessner and Johannes Mahl, Chemical Sciences Division, Lawrence Berkeley National Laboratory.
Scientists have now discovered a mechanism to detect electrons as they go from molecules to nanoparticles and back. Researchers can determine where electrons can readily go as well as when, where and why they become trapped. This information is critical for developing innovative material combinations.
The Impact
The research shows how a new experimental instrument that can track electrons as they flow between molecules and nanoparticles, converting sunlight into power or fuels. It turns out that zinc oxide, a common nanoparticle substance, temporarily slows electrons. The material then only allows electrons to flow along the nanoparticles’ very surface.
As a result, it is possible that the charges could be lost or the nanoparticle material will be damaged. Charges should ideally pass through nanoparticles without pausing and in a straight line. Researchers will be able to build better materials for converting sunlight into other types of energy if they can identify these electron transit bottlenecks.
Summary
A material must absorb light and transmit the light energy to electrons to convert sunlight into electricity or fuel. The electrons must then travel around to produce a current or allow chemical reactions to take place. Using molecules that are good at capturing sunlight and attaching them to substrates that are good at passing electrons around is one technique to accomplish both tasks.
Researchers had previously discovered that electrons could travel about more freely inside zinc oxide than they did in many other materials. Regardless, zinc oxide electrodes would not perform as well as electrodes constructed of other materials.
What exactly is going on? Researchers at the Advanced Light Source, a Department of Energy (DOE) Office of Science user’s facility, have been able to track the route of electrons from molecules to substrates and back using a technology called time-resolved X-Ray photoelectron spectroscopy.
Scientists discovered that electrons are trapped between both the molecules and the zinc oxide for a long period of time. When the electrons make the transition, the material continues to push them towards the substrate surface. The electrons are more readily trapped there than if they could go directly through the substrate bulk. This research sheds light on why zinc oxide substrates do not perform as well as expected. It also establishes a new testing methodology for future materials.
Funding
The Molecular, Atomic, and Optical Sciences Program of the DOE Office of Science, Office of Basic Energy Sciences, Geosciences, Chemical Sciences, and Biosciences Division provided funding for this research. The Liquid Sunlight Alliance, a DOE Fuels from Sunlight Hub financed by the DOE Office of Science, is funding two of the researchers.
The Advanced Light Source, a DOE Office of Science user facility, was employed in the study. The ALS Doctoral Fellowship in Residence Program, the Alexander von Humboldt Foundation and the Helmholtz Association, the Deutsche Forschungsgemeinschaft (e-conversion Cluster of Excellence), and the Helmholtz Association’s VI 419 all provided assistance to some of the researchers.
Journal Reference:
Neppl, S., et al. (2022) Nanoscale Confinement of Photo-Injected Electrons at Hybrid Interfaces. Journal of Physical Chemistry Letters. doi.org/10.1021/acs.jpclett.1c02648.