Reviewed by Mila PereraSep 19 2022
Using a gas-aggregation technique, two Pt-RE alloys in the nanoparticle form with even sizes, PtxY and PtxGd (x specifies changing stoichiometry or poorly characterized alloy structure), have been made ready from cluster sources. Their specific activities reached 14 mA cm−2, while the mass activities approached 4 A mgPt−1. These are among the highest values reported so far.
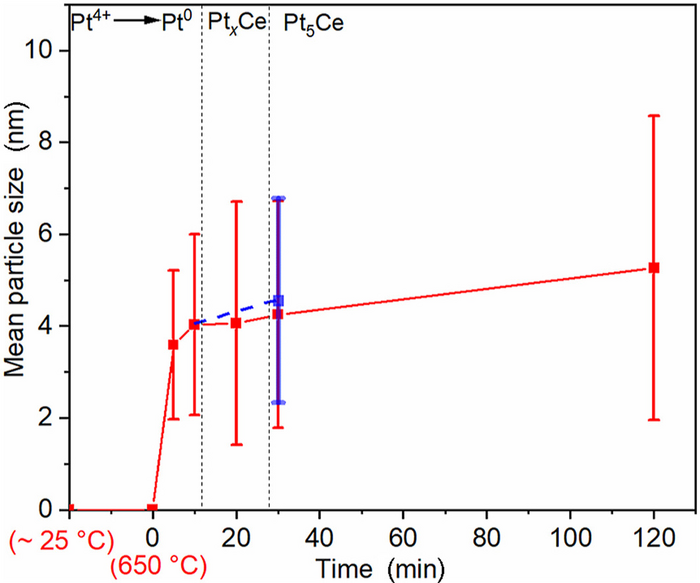 According to different mechanisms of the particle growth, the synthesis process is divided into three sequential periods, namely, Period 1, when the Pt4+ ions are reduced to Pt nanoparticles; Period 2, when Pt nanoparticles react with the Ce2(CN2)3 to form Pt5Ce; and Period 3, when the Pt5Ce particles grow further due to the prolonged heat-treatment at 650 °C. Art by Hu’s group. Image credit: Beijing Zhongke Journal Publishing Co. Ltd.
According to different mechanisms of the particle growth, the synthesis process is divided into three sequential periods, namely, Period 1, when the Pt4+ ions are reduced to Pt nanoparticles; Period 2, when Pt nanoparticles react with the Ce2(CN2)3 to form Pt5Ce; and Period 3, when the Pt5Ce particles grow further due to the prolonged heat-treatment at 650 °C. Art by Hu’s group. Image credit: Beijing Zhongke Journal Publishing Co. Ltd.
Dr. Yang Hu (Institute of Department of Energy Conversion and Storage, the Technical University of Denmark) and Dr. Qing-Feng Li (Institute of Department of Energy Conversion and Storage, Technical University of Denmark) led this study.
Pt-rare earth metal (RE) alloys — a family of catalysts — exhibit outstanding performance in acidic media toward the oxygen reduction reaction (ORR). For the comprehensive surface of bulk polycrystalline Pt5RE electrodes, the published specific activities at 0.9 V (vs. RHE) tested in 0.1 M HClO4 solution are in the range of 7 to 11 mA cm−2. This is 3.5–5.5 times more than that for the surface of polycrystalline Pt.
The PtxGd alloy particles reserved the mass activity of about 2.8 mA cmPt−1 after the accelerated stress test of 10,000 potential cycles in O2-saturated 0.1 M HClO4 from 0.6 to 1.0 V. Still, this is 2.8 times more active when compared to the pure Pt counterpart.
Nevertheless, interpreting these likely outcomes from bulk electrodes and model particles to a real-time catalyst has not yet been achieved, and so it has gained widespread research in the last decade.
The researchers look forward to synthesizing Pt-RE alloy catalysts on a considerably large scale and assessing their outstanding functioning in proton-exchange membrane (PEM) fuel cells, and they have achieved noteworthy advances.
Recently, Hu’s team created a worldwide, scalable chemical approach for synthesizing Pt-RE alloy catalysts supported by carbon. The vital process of synthesis is to heat a mixture of solid-state precursors in a reductive environment. Using this method, a collection of Pt-RE alloy catalysts, such as Pt3Y, Pt2Gd, and Pt5La, have been synthesized, and up to 10 g/batch production scale has been attained.
Both the stability and activity of the ORR are considerably affected by the size of Pt-RE alloy particles.
Earlier research on the model PtxGd and PtxY particles made from the cluster source showed that the ideal particle sizes are from 6 to 9 nm, which is larger than that of the pure Pt nanoparticles (i.e., 3 nm). The varying optimum sizes arise from the Pt-RE alloy particles' unique chemical and structural properties.
Rare earth metal ions exhibit extremely low standard reduction potentials, for example, –2.372 V for Y/Y3+. RE atoms are susceptible to being leached out from the surface area of the alloy particles to become a Pt overlayer when they come in contact with an acidic medium.
The overlayer is compressively strained due to the shorter Pt–Pt distance in the alloy particle’s core. On the Pt overlayer, this strain effect results in a little weakened binding energy of HO*, thereby increasing its activity toward the ORR.
This strain's effect is primarily based on the alloy core’s size. The smaller the particle, the weaker the effect.
Earlier works also demonstrated that Pt-RE alloy particles lesser than 3 nm lost most of the RE atoms once coming into contact in an acidic solution. Hence, Pt-RE alloy particles have to be significantly large, ideally over 6 nm, to gain good catalytic stability and activity.
However, big particles inevitably have tiny specific surface areas and, hence, low usage of the Pt atoms. Therefore, an ideal size range of 6–9 nm has been proposed for Pt-RE alloy particles for the ORR.
In this research, Hu and his team try to synthesize Pt-RE alloy catalysts with proposed optimal structures, an intermetallic Pt5RE phase with a particle size of 6–9 nm. Pt5Ce was selected as the phase for the target alloy since it is one of the most stable Pt-RE alloy structures indicated for the ORR, and Ce is one of the most plentiful and economic RE metals.
The two important factors in the industrial usage of the catalyst in PEM fuel cells are stability and price. Various synthesis conditions were tried, and a series of catalysts with a single Pt5Ce phase was successfully prepared.
Attempts were then made to customize the Pt5Ce particles’ sizes, which became this research's key challenge. They examined the growth model of the Pt5Ce particles in the complete synthesis process to achieve this task.
They then researched the consequence of two synthesis factors on the particle-growth process. They synthesized a Pt5Ce/C sample with a standard deviation of 1.3 nm and a mean particle size of 5.2 nm, exhibiting favorable ORR performance.
Journal Reference
Zhou, Q., et al. (2022) Tailoring the particle sizes of Pt5Ce alloy nanoparticles for the oxygen reduction reaction. Advanced Sensor and Energy Materials. doi.org/10.1016/j.asems.2022.100025.